Two common cofactors that are derived from the B vitamins, niacin and riboflavin, are nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD), respectively. The structure of NAD and FAD are shown below.

Figure 6.111 Structure of NAD1 upside down1. The atoms are circled to help orient this structure with Figure 6.113

Figure 6.112 Structure of FAD2. The nitrogens are circled to help orient this structure with Figure 6.114
Both of these cofactors can be reduced; NAD is reduced to form NADH, while FAD is reduced to form FADH2 as shown in the 2 figures below.

Figure 6.113 The reduction of NAD to form NADH3

Figure 6.114 The reduction of FAD4 to FADH2
An example of a mineral that serves as a cofactor is Fe2+ for proline and lysyl hydroxylases. We will discuss later in detail why vitamin C (ascorbic acid) is needed to reduce iron to Fe2+ so that it can serve as a cofactor for proline and lysyl hydroxylases.
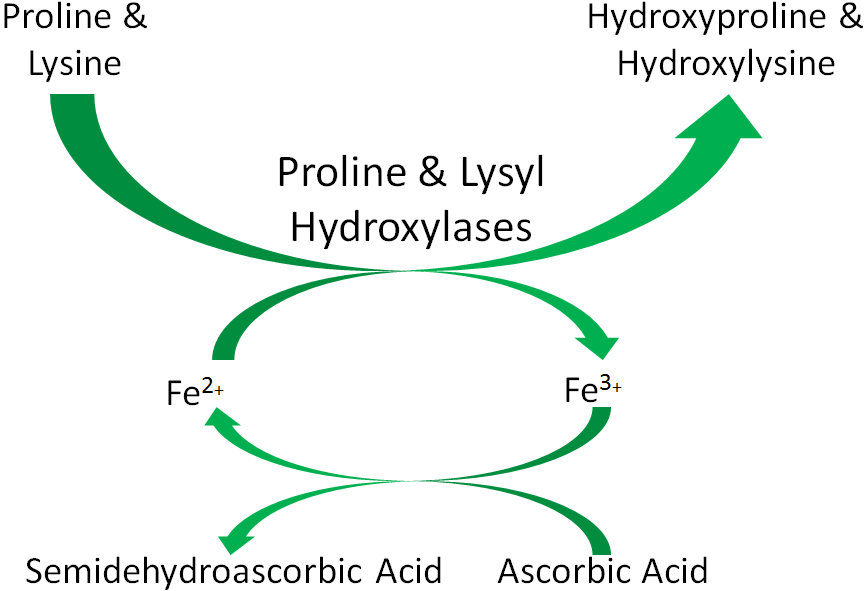
Figure 6.115 Iron (Fe2+) is a cofactor for proline and lysyl hydroxylases
References & Links
1. http://en.wikipedia.org/wiki/File:NAD%2B_phys.svg
2. http://en.wikipedia.org/wiki/File:Flavin_adenine_dinucleotide.png
3. http://en.wikipedia.org/wiki/File:NAD_oxidation_reduction.svg
4. http://en.wikipedia.org/wiki/File:FAD_FADH2_equlibrium.png
Candela Citations
- Kansas State University Human Nutrition Flexbook. Authored by: Brian Lindshield. Provided by: Kansas State University. Located at: http://goo.gl/vOAnR. License: CC BY: Attribution
