This assignment can be found in Google Docs: Chemistry for Majors Assignment: Atomic Structure and the Periodic Table
To make your own copy to edit:
- If you want a Google Doc: in the file menu of the open document, click “Make a copy.” This will give you your own Google Doc to work from.
- If you want a PDF or Word file: in the file menu of the open document, click “Download” and select the file type you would like to have (note: depending on the file type you select, the formatting could get jumbled).
Instructions
As you work these problems, consider and explain:
- What type of question is it?
- How do you know what type of question it is?
- What information are you looking for?
- What information do they give?
- How will you go about solving this?
- Show how to solve the problem.
- Be able to answer for a different reaction, number, set of conditions, etc.
Questions
- Which form of electromagnetic radiation has the longest wavelengths?
- A line in the spectrum of atomic mercury has a wavelength of 254 nm. When mercury emits a photon of light at this wavelength, what is the frequency of the light?
- Consider an atom traveling at 1% of the speed of light. The de Broglie wavelength is found to be 1.46 × 10–3 pm. Which element is this?
- What is the energy of a photon of blue light that has a wavelength of 453 nm?
- The four lines observed in the visible emission spectrum of hydrogen tell us that:
-
- The hydrogen molecules they came from have the formula H4.
- We could observe more lines if we had a stronger prism.
- There are four electrons in an excited hydrogen atom.
- Only certain energies are allowed for the electron in a hydrogen atom.
- The spectrum is continuous.
For questions 6–8, consider the following portion of the energy-level diagram for hydrogen:
n = 4 –0.1361 × 10–18 J n = 3 –0.2420 × 10–18 J n = 2 –0.5445 × 10–18 J n = 1 –2.178 × 10–18 J -
- For which of the following transitions does the light emitted have the longest wavelength?
- n = 4 to n = 3
- n = 4 to n = 2
- n = 4 to n = 1
- n = 3 to n = 2
- n = 2 to n = 1
- In the hydrogen spectrum, what is the wavelength of light associated with the n = 3 to n = 1 electron transition?
- When a hydrogen electron makes a transition from n = 3 to n = 1, which of the following statements is true?
- Energy is emitted.
- Energy is absorbed.
- The electron loses energy.
- The electron gains energy.
- The electron cannot make this transition.
- I, IV
- I, III
- II, III
- II, IV
- V
- Which of the following is a reasonable criticism of the Bohr model of the atom?
- It makes no attempt to explain why the negative electron does not eventually fall into the positive nucleus.
- It does not adequately predict the line spectrum of hydrogen.
- It does not adequately predict the ionization energy of the valence electron(s) for elements other than hydrogen.
- It does not adequately predict the ionization energy of the first energy level electrons for one-electron species for elements other than hydrogen.
- It shows the electrons to exist outside of the nucleus.
- The energy of the light emitted when a hydrogen electron goes from n = 2 to n = 1 is what fraction of its ground-state ionization energy?
- Which of the following is incorrect?
- The emission spectrum of hydrogen contains a continuum of colors.
- Diffraction produces both constructive and destructive interference.
- All matter displays both particle and wavelike characteristics.
- Niels Bohr developed a quantum model for the hydrogen atom.
- The lowest possible energy state of a molecule or atom is called its ground state.
- A gamma ray of wavelength 1.00 × 10–8 cm has enough energy to remove an electron from a hydrogen atom.
- Which of the following best describes an orbital?
- space where electrons are unlikely to be found in an atom
- space which may contain electrons, protons, and/or neutrons
- the space in an atom where an electron is most likely to be found
- small, walled spheres that contain electrons
- a single space within an atom that contains all electrons of that atom
- How many f orbitals have the value n = 3?
- If n = 2, how many orbitals are possible?
- Consider the following representation of a 2p-orbital:
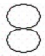
Which of the following statements best describes the movement of electrons in a p-orbital?- The electrons move along the outer surface of the p-orbital, similar to a “figure 8” type of movement.
- The electrons move within the two lobes of the p-orbital, but never beyond the outside surface of the orbital.
- The electrons are concentrated at the center (node) of the two lobes.
- The electrons are only moving in one lobe at any given time.
- The electron movement cannot be exactly determined.
- How many electrons in an atom can have the quantum numbers n = 3, l = 2?
- How many electrons can be contained in all of the orbitals with n = 4?
- Which of the following combinations of quantum numbers is not allowed?
- n = 1, l = 1, ml = 0, ms = 1/2
- n = 3, l = 0, ml = 0, ms = -1/2
- n = 2, l = 1, ml = -1, ms = 1/2
- n = 4, l = 3, ml = -2, ms = -1/2
- n = 4, l = 2, ml = 0, ms = 1/2
- Which of the following atoms or ions has three unpaired electrons?
- N
- O
- Al
- S2–
- Ti2+
- What is the electron configuration for the barium atom?
- What is the complete electron configuration of tin?
- Which of the following statements is true?
- The exact location of an electron can be determined if we know its energy.
- An electron in a 2s orbital can have the same n, l, and ml quantum numbers as an electron in a 3s orbital.
- Ni has two unpaired electrons in its 3d orbitals.
- In the buildup of atoms, electrons occupy the 4f orbitals before the 6s orbitals.
- Only three quantum numbers are needed to uniquely describe an electron.
- What is the statement that “the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals” known as?
- An element with the electron configuration [Xe] 6s24f145d7 would belong to which class on the periodic table?
- Ti has __________ in its d orbitals.
Candela Citations
CC licensed content, Original
- Authored by: Jessica Garber. Provided by: Tidewater Community College. License: CC BY: Attribution