What you’ll learn to do: Define atoms and elements
You’re probably familiar with the concept of atoms. They are the fundamental unit of matter—everything is made up of atoms, which come together in unique ways to form different things. Before you can understand chemical reactions, you must first understand the way that atoms work.
Over the years, scientists have used different models to visualize atoms as our understanding has changed. You may be familiar with a few of the models in Figure 1. In this course, we we largely use the Bohr model.

Figure 1. The evolution of atomic models over the years. As our understanding of atoms has evolved, the models we use to depict them have changed. The first model is the Thomson model, followed by the Rutherford model, the Bohr model, and the Heisenberg/Schrödinger model.
Learning Outcomes
- Identify the elements common in biological matter
- Explain the structure and components of an atom
- Identify the properties of elements given a periodic table
- Define the term isotope
Elements in Biological Matter
At its most fundamental level, life is made up of matter. Matter is any substance that occupies space and has mass. Elements are unique forms of matter with specific chemical and physical properties that cannot be broken down into smaller substances by ordinary chemical reactions. There are 118 elements, but only 92 occur naturally. The remaining elements are synthesized in laboratories and are unstable.
Each element is designated by its chemical symbol, which is a single capital letter or, when the first letter is already “taken” by another element, a combination of two letters. Some elements follow the English term for the element, such as C for carbon and Ca for calcium. Other elements’ chemical symbols derive from their Latin names; for example, the symbol for sodium is Na, referring to natrium, the Latin word for sodium.
The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N). In the non-living world, elements are found in different proportions, and some elements common to living organisms are relatively rare on the earth as a whole, as shown in Table 1. For example, the atmosphere is rich in nitrogen and oxygen but contains little carbon and hydrogen, while the earth’s crust, although it contains oxygen and a small amount of hydrogen, has little nitrogen and carbon. In spite of their differences in abundance, all elements and the chemical reactions between them obey the same chemical and physical laws regardless of whether they are a part of the living or non-living world.
| Table 1. Approximate Percentage of Elements in Living Organisms (Humans) Compared to the Non-living World | |||
|---|---|---|---|
| Element | Life (Humans) | Atmosphere | Earth’s Crust |
| Oxygen (O) | 65% | 21% | 46% |
| Carbon (C) | 18% | trace | trace |
| Hydrogen (H) | 10% | trace | 0.1% |
| Nitrogen (N) | 3% | 78% | trace |
Atoms
To understand how elements come together, we must first discuss the smallest component or building block of an element, the atom. An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, one gold atom has all of the properties of gold in that it is a solid metal at room temperature. A gold coin is simply a very large number of gold atoms molded into the shape of a coin and containing small amounts of other elements known as impurities. Gold atoms cannot be broken down into anything smaller while still retaining the properties of gold.

Figure 2. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
All atoms contain protons, electrons, and neutrons (Figure 2). The only exception is hydrogen (H), which is made of one proton and one electron.
A proton is a positively charged particle that resides in the nucleus (the core of the atom) of an atom and has a mass of 1 and a charge of +1.
Neutrons, like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom, which has a net zero charge.
An electron is a negatively charged particle that travels in the space around the nucleus. In other words, it resides outside of the nucleus. It has a negligible mass and has a charge of –1.
Because protons and neutrons each have a mass of 1, the mass of an atom is equal to the number of protons and neutrons of that atom. The number of electrons does not factor into the overall mass, because their mass is so small.
Build An Atom
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
Properties of Elements
Atomic Number and Mass
Each element has its own unique properties. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. The atomic number of an element is equal to the number of protons that element contains. The mass number is the number of protons plus the number of neutrons of that element. Therefore, it is possible to determine the number of neutrons by subtracting the atomic number from the mass number.
These numbers provide information about the elements and how they will react when combined. Different elements have different melting and boiling points, and are in different states (liquid, solid, or gas) at room temperature. They also combine in different ways. Some form specific types of bonds, whereas others do not. How they combine is based on the number of electrons present. Because of these characteristics, the elements are arranged into the periodic table of elements, a chart of the elements that includes the atomic number and relative atomic mass of each element. The periodic table also provides key information about the properties of elements (Figure 3)—often indicated by color-coding. The arrangement of the table also shows how the electrons in each element are organized and provides important details about how atoms will react with each other to form molecules.
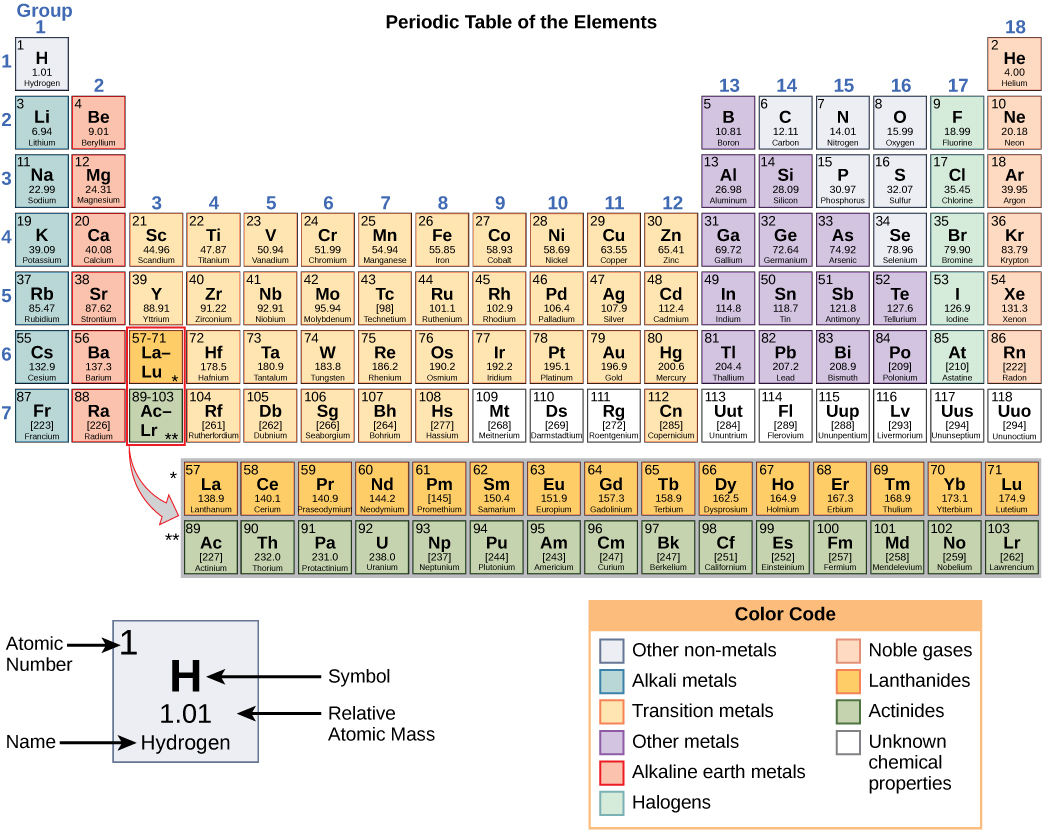
Figure 3. Arranged in columns and rows based on the characteristics of the elements, the periodic table provides key information about the elements and how they might interact with each other to form molecules. Most periodic tables provide a key or legend to the information they contain.
Practice Question
Complete the following table with information from the periodic table
| Name of Element | Symbol | Atomic Number | Atomic Mass (Round to the nearest whole number) |
|---|---|---|---|
| Beryllium | |||
| 8 | |||
| C | |||
| 32 | |||
| Na |
Element Interactions
How elements interact with one another depends on how their electrons are arranged and how many openings for electrons exist at the outermost region where electrons are present in an atom. Electrons exist at energy levels that form shells around the nucleus. The closest shell can hold up to two electrons. The closest shell to the nucleus is always filled first, before any other shell can be filled. Hydrogen has one electron; therefore, it has only one spot occupied within the lowest shell. Helium has two electrons; therefore, it can completely fill the lowest shell with its two electrons. If you look at the periodic table, you will see that hydrogen and helium are the only two elements in the first row. This is because they only have electrons in their first shell. Hydrogen and helium are the only two elements that have the lowest shell and no other shells.
The second and third energy levels can hold up to eight electrons. The eight electrons are arranged in four pairs and one position in each pair is filled with an electron before any pairs are completed.
Looking at the periodic table again (Figure 3), you will notice that there are seven rows. These rows correspond to the number of shells that the elements within that row have. The elements within a particular row have increasing numbers of electrons as the columns proceed from left to right. Although each element has the same number of shells, not all of the shells are completely filled with electrons. If you look at the second row of the periodic table, you will find lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne). These all have electrons that occupy only the first and second shells. Lithium has only one electron in its outermost shell, beryllium has two electrons, boron has three, and so on, until the entire shell is filled with eight electrons, as is the case with neon.
Isotopes
Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon-12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons and six neutrons) and an atomic number of 6 (which makes it carbon). Carbon-14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons and eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes.
Evolution in Action: Carbon Dating

Figure 4. The age of remains that contain carbon and are less than about 50,000 years old, such as this pygmy mammoth, can be determined using carbon dating.
Carbon-14 (14C) is a naturally occurring radioisotope that is created in the atmosphere by cosmic rays. This is a continuous process, so more 14C is always being created. As a living organism develops, the relative level of 14C in its body is equal to the concentration of 14C in the atmosphere. When an organism dies, it is no longer ingesting 14C, so the ratio will decline. 14C decays to 14N by a process called beta decay; it gives off energy in this slow process.
After approximately 5,730 years, only one-half of the starting concentration of 14C will have been converted to 14N. The time it takes for half of the original concentration of an isotope to decay to its more stable form is called its half-life. Because the half-life of 14C is long, it is used to age formerly living objects, such as fossils. Using the ratio of the 14C concentration found in an object to the amount of 14C detected in the atmosphere, the amount of the isotope that has not yet decayed can be determined. Based on this amount, the age of the fossil can be calculated to about 50,000 years (Figure 4). Isotopes with longer half-lives, such as potassium-40, are used to calculate the ages of older fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years.
Check Your Understanding
The number and arrangement of ________ controls how elements interact.
- protons
- electrons
- neutrons
Show Answer
electrons
Candela Citations
- Introduction to Atoms and Elements. Authored by: Shelli Carter and Lumen Learning. Provided by: Lumen Learning. License: CC BY: Attribution
- Evolution of atomic models infographic. Authored by: Ville Takanen. Located at: https://commons.wikimedia.org/wiki/File:Evolution_of_atomic_models_infographic.svg. License: CC BY: Attribution
- Biology. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.8. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.8
- Concepts of Biology. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.25. License: CC BY: Attribution. License Terms: http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.25
- Build an Atom. Provided by: PhET. Located at: https://phet.colorado.edu/en/simulation/build-an-atom. License: CC BY: Attribution
- Carbon Dating. Authored by: Bill Faulkner/NPS. Located at: https://www.nps.gov/chis/learn/historyculture/pygmymammoth.htm. License: Public Domain: No Known Copyright