Learning Objectives
By the end of this section, you will be able to:
- Explain metabolic pathways
- State the laws of thermodynamics
- Explain the difference between kinetic and potential energy
- Describe endergonic and exergonic reactions
- Discuss the importance of enzyme function

Figure 1. Ultimately, most life forms get their energy from the sun. Plants use photosynthesis to capture sunlight, and herbivores eat the plants to obtain energy. Carnivores eat the herbivores, and eventual decomposition of plant and animal material contributes to the nutrient pool.
Metabolic Pathways
Sugar metabolism is a classic example of one of the many cellular processes that use and produce energy. Living things consume sugar as a major energy source due to the high energy stored within their bonds. During photosynthesis, plants use solar energy to convert carbon dioxide gas (CO2) into sugar molecules (like glucose: C6H12O6). Oxygen is produced as a waste product. This reaction is summarized as:
It requires energy input to proceed. During photosynthesis, energy is provided by adenosine triphosphate (ATP), the primary energy currency of all cells. Just as the dollar is used as currency to buy goods, cells use ATP molecules as energy currency to perform work. During cellular respiration, glucose is used as an energy source. It can be summarized by the reverse reaction to photosynthesis. In this reaction, oxygen is consumed and carbon dioxide is released as a waste product. The reaction is summarized as:
Although simplified, both of these reactions involve many steps. A metabolic pathway is very organized series of linked chemical reactions. Two opposite processes are involved. Anabolic pathways require energy input in order to produce large molecules(polymers). Catabolic pathways release energy by breaking down polymers into their smaller molecules(monomers). Consequently, metabolism is composed of synthesis (anabolism) and degradation (catabolism) (Figure 2).

Figure 2. Catabolic pathways are those that generate energy by breaking down larger molecules. Anabolic pathways are those that require energy to synthesize larger molecules. Both types of pathways are required for maintaining the cell’s energy balance.
Energy
Thermodynamics refers to the study of energy. When heating a pot of water on the stove, the system includes the stove, the pot, and the water. Energy is transferred within the system (between the stove, pot, and water). There are two types of systems: open and closed. In an open system, energy can be exchanged with its surroundings. The stovetop system is open because heat can be lost to the air. A closed system cannot exchange energy with its surroundings.
Biological organisms are open systems. Energy is exchanged between them and their surroundings. Organisms use solar energy to perform photosynthesis. An organism can also consume molecules and release energy to the environment by doing work releasing heat. Like all things in the physical world, energy is subject to physical laws. The laws of thermodynamics govern the transfer of energy in and among all systems in the universe.
In general, energy is defined as the ability to do work, or to create some kind of change. Energy exists in different forms. Electrical energy, light energy, and heat energy are all different types of energy. To appreciate the way energy flows into and out of biological systems, it is important to understand two of the physical laws that govern energy.
Thermodynamics
The first law of thermodynamics states that energy cannot be created or destroyed but can be transferred or transformed. In other words, there has always been, and always will be, exactly the same amount of energy in the universe. The transfers and transformations of energy take place constantly. Light bulbs transform electrical energy into light and heat energy. Gas stoves transform chemical energy from natural gas into heat energy. Plants perform one of the most biologically useful energy transformations on earth. Plants convert solar energy to chemical energy stored within organic molecules. Some examples of energy transformations are shown in (Figure 3).
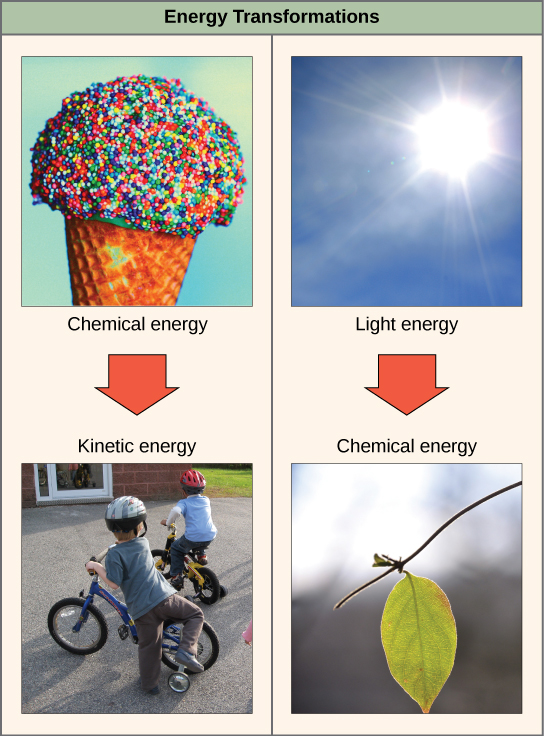
Figure 3. Shown are some examples of energy transferred and transformed from one system to another and from one form to another. The food we consume provides our cells with the energy required to carry out bodily functions, just as light energy provides plants with the means to create the chemical energy they need. (credit “ice cream”: modification of work by D. Sharon Pruitt; credit “kids”: modification of work by Max from Providence; credit “leaf”: modification of work by Cory Zanker)
The challenge for all living organisms is to obtain energy from their surroundings in forms that they can transfer or transform into usable energy to do work. Living cells have evolved to meet this challenge. Chemical energy stored within organic molecules is transferred and transformed through a series of cellular chemical reactions into energy within molecules of ATP. Energy in ATP molecules is easily accessible to do work. Cellular work includes building complex molecules, transporting materials, powering the motion of flagella, and contracting muscle fibers to movement.
All energy transfers and transformations are never completely efficient. The second law of thermodynamics states that in every energy transfer, some amount of energy is lost in a less usable form. In most cases, this form is heat energy. Thermodynamically, heat energy is defined as the energy transferred from one system to another that is not work. When a light bulb is turned on, some of the energy being converted from electrical energy into light energy is lost as heat energy. During many metabolic reactions within a cell, some energy is lost as heat energy.
An important concept in physical systems is that of order and disorder. The more energy that is lost by a system to its surroundings, the less ordered and more random the system will be. Scientists refer to the measure of randomness or disorder within a system as entropy. High entropy means high disorder and low energy. Molecules and chemical reactions have varying entropy. Entropy increases as molecules at a high concentration in one place diffuse and spread out. Living things are highly ordered, requiring constant energy input to be maintained in a state of low entropy.
Kinetic and Potential Energy
When an object is in motion, there is energy associated with that object. Think of a wrecking ball. Even a slow-moving wrecking ball can do a great deal of damage. Energy associated with objects in motion is called kinetic energy (Figure 4). A speeding bullet, a jogger, and the wrecking ball all have kinetic energy.

Figure 4. Still water has potential energy; moving water, such as in a waterfall or a rapidly flowing river, has kinetic energy. (credit “dam”: modification of work by “Pascal”/Flickr; credit “waterfall”: modification of work by Frank Gualtieri)
What if that same wrecking ball is lifted two stories above ground with a crane? If the suspended wrecking ball is not moving, is there energy associated with it? The answer is yes. The energy that was required to lift the wrecking ball did not disappear, but is now stored in the wrecking ball by virtue of its position and the force of gravity acting on it. This type of energy is called potential energy (Figure 4). If the ball were to fall, the potential energy would be transformed into kinetic energy until all of the potential energy was exhausted when the ball came to rest. Wrecking balls swing like a pendulum; through the swing, there is a constant change of potential energy (highest at the top of the swing) to kinetic energy (highest at the bottom of the swing). Other examples of potential energy include the energy of water held behind a dam or a person about to skydive out of an airplane.
Potential energy is not only associated with the location of matter, but also with its structure. Even a spring on the ground has potential energy if it is compressed. On a molecular level, the molecular bonds that hold the atoms together exist in a particular structure that has potential energy. The fact that energy can be released by the breakdown of certain chemical bonds implies that those bonds have potential energy. In fact, there is potential energy stored within the bonds of all the food molecules we eat, which is eventually harnessed for use. This is because these bonds can release energy when broken. The type of potential energy that exists within chemical bonds is called chemical energy. Chemical energy is responsible for providing living cells with energy from food. The release of energy occurs when the molecular bonds within food molecules are broken.
Concept in Action
Exergonic vs. Endergonic Reactions
How can the energy released from one reaction be compared to that of another reaction? A measurement of free energy is used to determine these energy transfers. According to the second law of thermodynamics, all energy transfers involve the loss of some amount of energy in an less usable form. Free energy refers to the energy associated with a chemical reaction that is available after accounting for any loss. In other words, free energy is usable energy, or energy that is available to do work.
If energy is released during a chemical reaction, then the change in free energy, signified as ∆G (delta G), will be a negative number. A negative change in free energy means that the products of the reaction have less free energy than the reactants, because they release some free energy during the reaction. Reactions that have a negative change in free energy and consequently release free energy are called exergonic reactions. Exergonic means energy is exiting the system. These reactions are also referred to as spontaneous reactions, and their products have less stored energy than the reactants. An important distinction must be drawn between the term spontaneous and the idea of a chemical reaction occurring immediately. Contrary to the everyday use of the term, a spontaneous reaction is not one that suddenly or quickly occurs. The rusting of iron is an example of a spontaneous reaction that occurs slowly, little by little, over time.
If a chemical reaction absorbs energy rather than releases energy , then the ∆G will be a positive value. In this case, the products have more free energy than the reactants. Thus, the products of these reactions can be thought of as energy-storing molecules. These chemical reactions are called endergonic reactions and they are not spontaneous. An endergonic reaction will not take place on its own without the addition of free energy.
Art Connection
Look at each of the processes shown in Figure 5 and decide if it is endergonic or exergonic.

Figure 5. Shown are some examples of endergonic processes (ones that require energy) and exergonic processes (ones that release energy). (credit a: modification of work by Natalie Maynor; credit b: modification of work by USDA; credit c: modification of work by Cory Zanker; credit d: modification of work by Harry Malsch)
There is another important concept that must be considered regarding exergonic and endergonic reactions. Exergonic reactions require a small amount of energy input to get started before they can proceed with their energy-releasing steps. These reactions have a net release of energy, but still require some energy input in the beginning. This small amount of energy input necessary for all chemical reactions to occur is called the activation energy.
Concept in Action
Watch this animation of the move from free energy to transition state of the reaction.
Enzymes

Figure 6. Enzymes lower the activation energy of the reaction but do not change the free energy of the reaction.
Catalyst are substances that allow a reaction to occur at a faster rate. Enzymes are the catalyst for biochemical reactions . Most enzymes are proteins and when present, lower the energy needed for a reaction to occur. This energy is referred to as activation energy. Most reactions critical to living cell happen too slowly at normal temperatures to be of any use to a cell. Without enzymes to speed up these reactions, life could not continue. Enzymes bind to the reactant molecules and allow bonds to break and form more easily. Enzymes do not change whether a reaction is exergonic or endergonic. They do not change the free energy of the reactants or products, but reduce the activation energy(Figure 6). Since the enzyme remain virtually unchanged, once the reaction is complete, the enzyme is able to participate in other reactions.
When an enzyme is involved in a chemical reaction, the reactants are referred to as substrates. The specific area where the enzyme and substrate connect is called the active site. The active site is where the “action” happens.
Since enzymes are proteins environmental factors are highly involved in their reaction rates. Two major factors that have an effect on enzyme activity include:
(1) temperature changes
(2) changes in pH
Temperature increases generally increase reaction rates of many reactions. However, temperatures outside of an optimal range can greatly affect whether an enzyme will be involved in a reaction. As temperatures rise, enzyme activity will rise until the optimal temperature is reached for the enzyme. If the temperature continues to rise, the enzyme, being a protein, will exhibit an irreversible change in shape or denature. Once the enzyme changes shape, it will no longer attach to the substrate and the reaction will not proceed. This is another indication of the importance of how structure is important to function in all living things.
Enzymes are also suited to function best within a narrow pH range. As with temperature, enzyme have a preferred optimal pH range. Most human enzymes work best at pH ranges between 6-8. Once passed the optimal range, the enzyme will denature, just was with temperature.
INDUCED-FIT MODEL
For many years, scientists thought that enzyme-substrate binding took place in a simple “lock and key” fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. Current research supports a model known as induced-fit(Figure 7). When an enzyme binds to its substrate, an enzyme-substrate complex is formed. The enzyme can alter the shape of the active site SLIGHTLY to ensure an good fit for the reaction to continue. Once the product is produced, the enzyme will change back to its original shape to be used in another reaction. For this reason, enzymes are only needed in small amounts for a reaction to occur.
Concept in Action
View this animation of induced fit.

Figure 7. The induced-fit model is an adjustment to the lock-and-key model and explains how enzymes and substrates undergo dynamic modifications during the transition state to increase the affinity of the substrate for the active site.
Cellular needs and conditions constantly vary from cell to cell and even change within individual cells over time. The required enzymes of stomach cells differ from those of skin cells, blood cells, and nerve cells. A digestive organ cell works much harder to process and break down nutrients closely following a meal compared with many hours after a meal. As these cellular demands and conditions vary, so must the amounts and functionality of different enzymes. The relative amounts and functioning of enzyme variety within a cell ultimately determine which reactions will proceed and at what rates. This determination is tightly controlled in cells. In certain cellular environments, enzyme activity is partly controlled by environmental factors as mentioned before with temperature and pH.
Enzymes can be prevented from entering a reaction. There are two common types of inhibition:
(1) competitive inhibition – an inhibitor molecule is similar enough to a substrate that it can bind to the active site and simply block the substrate from binding; the inhibitor molecule competes with the substrate for binding to the active site.
(2) noncompetitive inhibition – an inhibitor molecule binds to the enzyme in a location other than the active site, called an allosteric site; blocks substrate binding to the active site
two ways noncompetitive inhibition occurs:(Figure 8) (a) allosteric inhibition – inhibitor binds to enzyme inducing a conformational change reducing affinity for substrate; all active sites changed slightly; (b) allosteric activation – bind to locations on an enzyme away from the active site; induce a conformational change increasing affinity of the enzyme’s active site(s) for its substrate(s)

Figure 8. Allosteric inhibition works by indirectly inducing a conformational change to the active site such that the substrate no longer fits. In contrast, in allosteric activation, the activator molecule modifies the shape of the active site to allow a better fit of the substrate.
Careers in Action
Pharmaceutical Drug Developer

Figure 7. Have you ever wondered how pharmaceutical drugs are developed? (credit: Deborah Austin)
Enzymes are key components of metabolic pathways. Understanding how enzymes work and how they can be regulated are key principles behind the development of many of the pharmaceutical drugs on the market today. Biologists working in this field collaborate with other scientists to design drugs.
Consider statins for example—statins is the name given to one class of drugs that can reduce cholesterol levels. These compounds are inhibitors of the enzyme HMG-CoA reductase, which is the enzyme that synthesizes cholesterol from lipids in the body. By inhibiting this enzyme, the level of cholesterol synthesized in the body can be reduced. Similarly, acetaminophen, popularly marketed under the brand name Tylenol, is an inhibitor of the enzyme cyclooxygenase. While it is used to provide relief from fever and inflammation (pain), its mechanism of action is still not completely understood.
How are drugs discovered? One of the biggest challenges in drug discovery is identifying a drug target. A drug target is a molecule that is literally the target of the drug. In the case of statins, HMG-CoA reductase is the drug target. Drug targets are identified through painstaking research in the laboratory. Identifying the target alone is not enough. Scientists need to know how the target acts inside the cell and which reactions go awry in the case of disease. Once the target and the pathway are identified, then the actual process of drug design begins. In this stage, chemists and biologists work together to design and synthesize molecules that can block or activate a particular reaction. But, this is only the beginning: If and when a drug prototype is successful in performing its function, it is subjected to many tests from in vitro experiments to clinical trials before it can get approval from the U.S. Food and Drug Administration to be on the market.
Many enzymes do not work optimally, or even at all, unless bound to other specific non-protein helper molecules. They may bond either temporarily through ionic or hydrogen bonds, or permanently through stronger covalent bonds. Binding to these molecules promotes optimal shape and function of their respective enzymes. Two examples of these types of helper molecules are cofactors and coenzymes. Cofactors are inorganic ions such as ions of iron and magnesium. Coenzymes are organic helper molecules, those with a basic atomic structure made up of carbon and hydrogen. Like enzymes, these molecules participate in reactions without being changed themselves and are ultimately recycled and reused. Vitamins are the source of coenzymes. Some vitamins are the precursors of coenzymes and others act directly as coenzymes. Vitamin C is a direct coenzyme for multiple enzymes that take part in building the important connective tissue, collagen. Therefore, enzyme function is, in part, regulated by the abundance of various cofactors and coenzymes, which may be supplied by an organism’s diet or, in some cases, produced by the organism.
Feedback Inhibition in Metabolic Pathways
Molecules can regulate enzyme function in many ways. What are these molecules and where do they come from? What other molecules in the cell provide enzymatic regulation? Perhaps the most relevant source of regulatory molecules are the products of the reactions themselves. In a most efficient and elegant way, cells have evolved to use the products of their own reactions for feedback inhibition of enzyme activity. Feedback inhibition involves the use of a reaction product to regulate its own further production (Figure 9). The cell responds to an abundance of the products by slowing down production during anabolic or catabolic reactions. Such reaction products may inhibit the enzymes that catalyzed their production through the mechanisms described above.

Figure 9. Metabolic pathways are a series of reactions catalyzed by multiple enzymes. Feedback inhibition, where the end product of the pathway inhibits an upstream process, is an important regulatory mechanism in cells.
Section Summary
Cells perform life functions through various chemical reactions. Metabolism refers to the combination of chemical reactions that take place. Anabolic processes build complex molecules out of simpler ones and require energy. Catabolic reactions break down complex chemicals into simpler ones and are associated with energy release.
Entropy is a measure of the disorder in a system. The physical laws that describe the transfer of energy are the laws of thermodynamics. The first law states that the total amount of energy in the universe is constant. The second law of thermodynamics states that every energy transfer involves some loss of energy in an unusable form, such as heat energy. Energy comes in different forms: kinetic and potential. The change in free energy of a reaction can be negative (releases energy, exergonic) or positive (consumes energy, endergonic). All reactions require an initial input of energy to proceed, called the activation energy.
Enzymes are chemical catalysts that speed up chemical reactions by lowering their activation energy. Enzymes have an active site with a unique chemical environment that fits particular chemical reactants for that enzyme, called substrates. Enzymes and substrates are thought to bind according to an induced-fit model. Enzyme action is regulated to conserve resources and respond optimally to the environment.
Additional Self Check Questions
2. Does physical exercise to increase muscle mass involve anabolic and/or catabolic processes? Give evidence for your answer.
3. Explain the difference between a spontaneous reaction and one that occurs instantaneously.
Answers
1. A compost pile decomposing is an exergonic process. A baby developing from a fertilized egg is an endergonic process. Tea dissolving into water is an exergonic process. A ball rolling downhill is an exergonic process.
2. Physical exercise involves both anabolic and catabolic processes. Body cells break down sugars to provide ATP to do the work necessary for exercise, such as muscle contractions. This is catabolism. Muscle cells also must repair muscle tissue damaged by exercise by building new muscle. This is anabolism.
3. A spontaneous reaction is one that has a negative ∆G and thus releases energy. However, a spontaneous reaction need not occur quickly or suddenly like an instantaneous reaction. It may occur over long periods of time due to a large energy of activation, which prevents the reaction from occurring quickly.
Glossary
activation energy: the amount of energy necessary for reactions to occur
anabolic: describes the pathway that requires a net energy input to synthesize complex molecules from simpler ones
catabolic: describes the pathway in which complex molecules are broken down into simpler ones, yielding energy as an additional product of the reaction
endergonic: describes a chemical reaction that results in products that store more chemical potential energy than the reactants
feedback inhibition: a mechanism of enzyme activity regulation in which the product of a reaction inhibits an enzyme for an earlier step in the reaction series
kinetic energy: the type of energy associated with objects in motion
potential energy: the type of energy that refers to the potential to do work
Candela Citations
- Concepts of Biology. Authored by: Open Stax. Located at: http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@8.10:1/Concepts_of_Biology. License: CC BY: Attribution