Learning Objectives
By the end of this section, you will be able to:
- Describe the properties of water that are critical to maintaining life
Do you ever wonder why scientists spend time looking for water on other planets? It is because water is essential to life; even minute traces of it on another planet can indicate that life could or did exist on that planet. Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 70 percent of your body is made up of water. Without it, life simply would not exist.
PROPERTIES OF WATER
Water Is Polar

Figure 1. As this macroscopic image of oil and water show, oil is a nonpolar compound and, hence, will not dissolve in water. Oil and water do not mix. (credit: Gautam Dogra)
As mentioned earlier, the hydrogen and oxygen atoms within water molecules form polar covalent bonds. The electrons within the bond are shared unequally. There is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom but no overall charge to the molecule as a whole. Because of these charges, the slightly positive hydrogen atoms repel each other and form the unique shape seen in Figure 1. Each water molecule attracts other water molecules because of the positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with water, it can dissolve in water and is referred to as hydrophilic (“water-loving”). Hydrogen bonds are not readily formed with nonpolar substances like oils and fats (Figure 1). These nonpolar compounds are hydrophobic (“water-fearing”) and will not dissolve in water.
Water Stabilizes Temperature
The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances. Temperature is a measure of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules. Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy and temperature changes only minimally. This means that water moderates temperature changes within organisms and in their environments. As energy input continues, the balance between hydrogen-bond formation and destruction swings toward the destruction side. More bonds are broken than are formed. This process results in the release of individual water molecules at the surface of the liquid (such as a body of water, the leaves of a plant, or the skin of an organism) in a process called evaporation. Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of energy and takes heat away from the body.
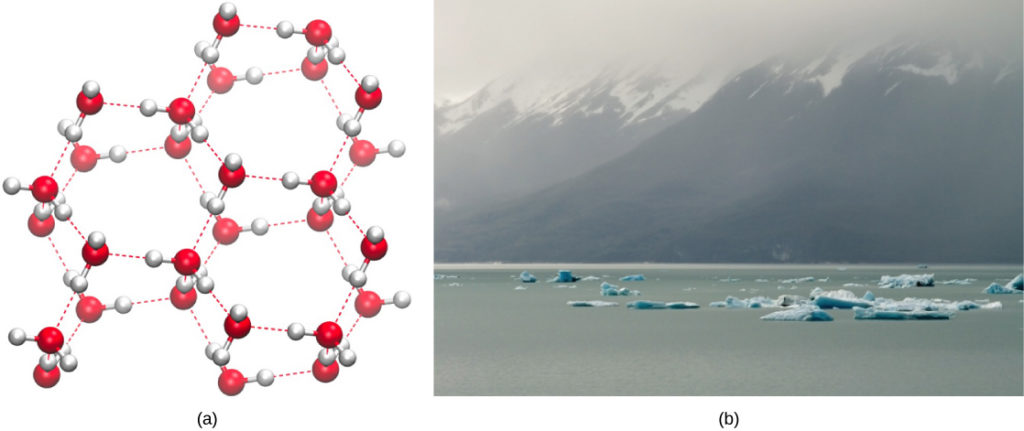
Figure 2. (a) The lattice structure of ice makes it less dense than the freely flowing molecules of liquid water. Ice’s lower density enables it to (b) float on water. (credit a: modification of work by Jane Whitney; credit b: modification of work by Carlos Ponte)
Water Is an Excellent Solvent
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent—a substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. In the case of table salt (NaCl) mixed in water (Figure 3), the sodium and chloride ions separate, or dissociate, in the water. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecule. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
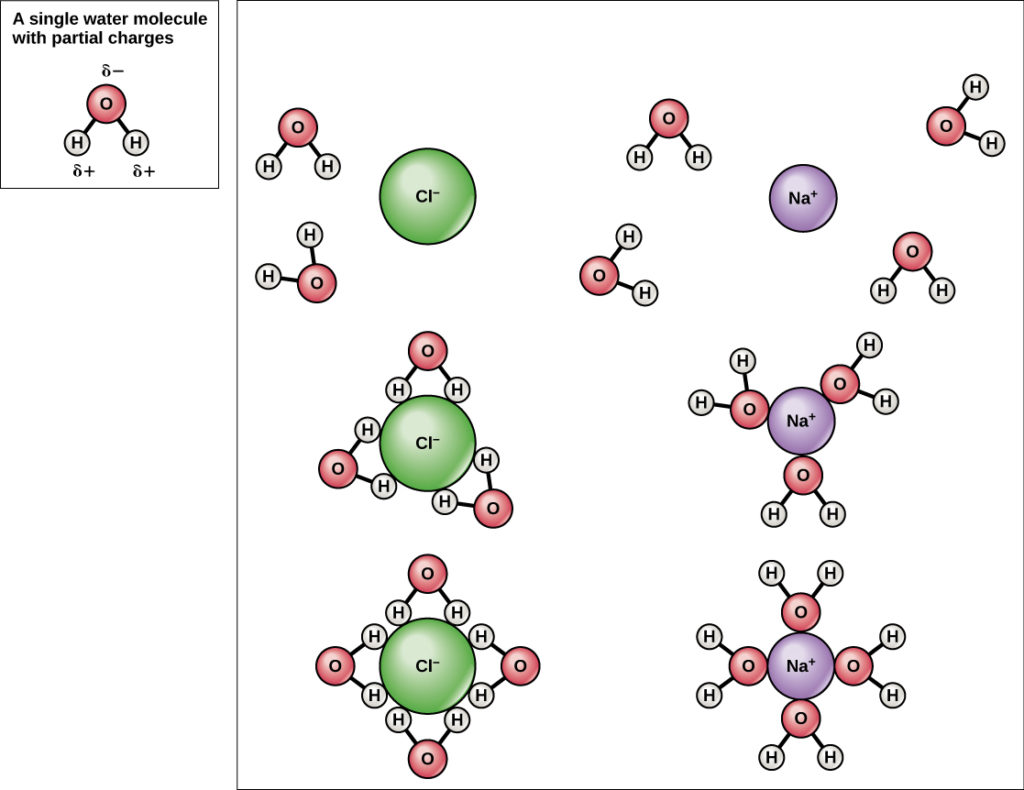
Figure 3. When table salt (NaCl) is mixed in water, spheres of hydration form around the ions.
Water Is Cohesive/Adhesive

Figure 4. The weight of a needle on top of water pulls the surface tension downward; at the same time, the surface tension of the water is pulling it up, suspending the needle on the surface of the water and keeping it from sinking. Notice the indentation in the water around the needle. (credit: Cory Zanker)
Have you ever filled up a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water actually forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. When you drop a small scrap of paper onto a droplet of water, the paper floats on top of the water droplet, although the object is denser (heavier) than the water. This occurs because of the surface tension that is created by the water molecules. Cohesion and surface tension keep the water molecules intact and the item floating on the top. It is even possible to “float” a steel needle on top of a glass of water if you place it gently, without breaking the surface tension (Figure 4).
These cohesive forces are also related to the water’s property of adhesion, or the attraction between water molecules and other molecules. This is observed when water “climbs” up a straw placed in a glass of water. You will notice that the water appears to be higher on the sides of the straw than in the middle. This is because the water molecules are attracted to the straw and therefore adhere to it.
Cohesive and adhesive forces are important for sustaining life. Because of these forces, water can flow up from the roots to the tops of plants to feed the plant.
Concept in Action
To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water!
Acids and Bases

Figure 5. The pH scale measures the amount of hydrogen ions (H+) in a substance. (credit: modification of work by Edward Stevens)
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper to test how much acid or base exists in solutions. This paper has been treated with a natural water-soluble dye so it can be used as a pH indicator. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 5). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10. So, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100 and so on. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0. Anything below 7(ranging from 0 to 6) is acidic, and anything above 7 (from 7 to 14) is alkaline(basic). Human blood is slightly alkaline (pH = 7.4), while the environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).
Acids are substances that provide hydrogen ions (H+) and lower pH, whereas bases provide hydroxide ions (OH–) and raise pH. The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water. Bases are those substances that readily donate OH–. The OH– ions combine with H+ to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH– rapidly when placed in water, thereby raising the pH.
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will break down. Deviation outside of the pH range can induce coma or even cause death.
So how is it that we can take in acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the pH of the body carefully maintained in this narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Section Summary
Water has many properties that are critical to maintaining life. It is polar which allows ions and other polar molecules to dissolve in water. Water is an excellent solvent. The hydrogen bonds between water molecules give water the ability to hold heat better than many other substances. As the temperature rises, the hydrogen bonds between water continually break and reform, allowing for the overall temperature to remain stable, although increased energy is added to the system. Water’s cohesive forces allow for the property of surface tension. All of these unique properties of water are important in the chemistry of living organisms.
The pH of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen ions is acidic and has a low pH value. A solution with a high number of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain constant pH conditions.
Self Check Questions
- Why can some insects walk on water?
- Explain why water is an excellent solvent.
Answers
- Some insects can walk on water, although they are heavier (denser) than water, because of the surface tension of water. Surface tension results from cohesion, or the attraction between water molecules at the surface of the body of water [the liquid-air (gas) interface].
- Water molecules are polar, meaning they have separated partial positive and negative charges. Because of these charges, water molecules are able to surround charged particles created when a substance dissociates. The surrounding layer of water molecules stabilizes the ion and keeps differently charged ions from reassociating, so the substance stays dissolved.
Candela Citations
- Concepts of Biology. Authored by: Open Stax. Located at: http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@8.10:1/Concepts_of_Biology. License: CC BY: Attribution