The Born-Haber Cycle
It is not possible to measure lattice energies directly. However, the lattice energy can be calculated using the equation given in the previous section or by using a thermochemical cycle. The Born-Haber cycle is an application of Hess’s law that breaks down the formation of an ionic solid into a series of individual steps:
- [latex]\Delta{H}_{\text{f}}^{\circ},[/latex] the standard enthalpy of formation of the compound
- IE, the ionization energy of the metal
- EA, the electron affinity of the nonmetal
- [latex]\Delta{H}_{s}^{\circ},[/latex] the enthalpy of sublimation of the metal
- D, the bond dissociation energy of the nonmetal
- ΔHlattice, the lattice energy of the compound
Figure 2 diagrams the Born-Haber cycle for the formation of solid cesium fluoride.
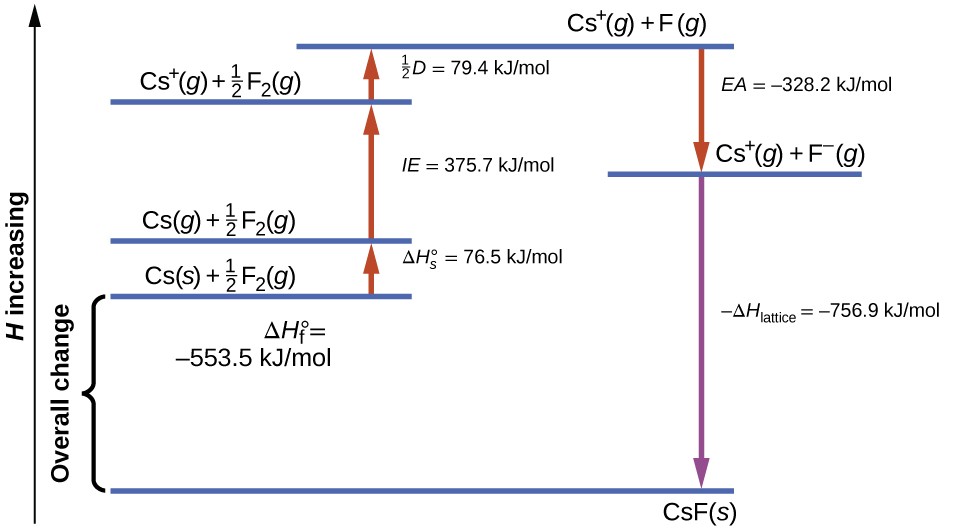
Figure 2. The Born-Haber cycle shows the relative energies of each step involved in the formation of an ionic solid from the necessary elements in their reference states.
We begin with the elements in their most common states, Cs(s) and F2(g). The [latex]\Delta{H}_{s}^{\circ}[/latex] represents the conversion of solid cesium into a gas, and then the ionization energy converts the gaseous cesium atoms into cations. In the next step, we account for the energy required to break the F-F bond to produce fluorine atoms. Converting one mole of fluorine atoms into fluoride ions is an exothermic process, so this step gives off energy (the electron affinity) and is shown as decreasing along the y-axis. We now have one mole of Cs cations and one mole of F anions. These ions combine to produce solid cesium fluoride. The enthalpy change in this step is the negative of the lattice energy, so it is also an exothermic quantity. The total energy involved in this conversion is equal to the experimentally determined enthalpy of formation, [latex]\Delta{H}_{\text{f}}^{\circ},[/latex] of the compound from its elements. In this case, the overall change is exothermic.
Hess’s law can also be used to show the relationship between the enthalpies of the individual steps and the enthalpy of formation. Table 3 shows this for cesium chloride, CsCl2.
| Table 3 | |
|---|---|
| Enthalpy of sublimation of Cs(s) | [latex]\text{Cs}\left(s\right)\rightarrow\text{Cs}\left(g\right)\Delta H=\Delta{H}_{s}^{\circ}=76.5\text{kJ}[/latex] |
| One-half of the bond energy of Cl2 | [latex]\frac{1}{2}{\text{Cl}}_{2}\text{(}g\text{)}\rightarrow\text{Cl}\left(g\right)\Delta H=\frac{1}{2}D=122\text{kJ}[/latex] |
| Ionization energy of Na(g) | [latex]\text{Na}\left(g\right)\rightarrow{\text{Na}}^{\text{+}}\text{(}g\text{)}+{\text{e}}^{-}\Delta H=IE=496\text{kJ}[/latex] |
| Negative of the electron affinity of Cl | [latex]\text{Cl}\left(g\right)+{\text{e}}^{-}\rightarrow{\text{Cl}}^{-}\text{(}g\text{)}\Delta H=-EA=-368\text{kJ}[/latex] |
| Negative of the lattice energy of NaCl(s) | [latex]{\text{Na}}^{\text{+}}\text{(}g\text{)}+{\text{Cl}}^{-}\text{(}g\text{)}\rightarrow\text{NaCl}\left(s\right)\Delta H=-{\Delta{H}}_{\text{lattice}}=?[/latex] |
| Enthalpy of formation of NaCl(s), add steps 1–5 | [latex]\begin{array}{l}\Delta H=\Delta{H}_{f}^{\circ}=\Delta{H}_{s}^{\circ}+\frac{1}{2}D+IE+\left(-EA\right)+\left(-{\Delta{H}}_{\text{lattice}}\right)\\ \text{Na}\left(s\right)+\frac{1}{2}{\text{Cl}}_{2}\left(g\right)\rightarrow\text{NaCl}\left(s\right)=-411\text{kJ}\end{array}[/latex] |
Thus, the lattice energy can be calculated from other values. For cesium chloride, using this data, the lattice energy is:
[latex]\Delta{H}_{\text{lattice}}=\left(411+109+122+496+368\right)\text{kJ}=770\text{kJ}[/latex]
The Born-Haber cycle may also be used to calculate any one of the other quantities in the equation for lattice energy, provided that the remainder is known. For example, if the relevant enthalpy of sublimation [latex]\Delta{H}_{s}^{\circ},[/latex] ionization energy (IE), bond dissociation enthalpy (D), lattice energy ΔHlattice, and standard enthalpy of formation [latex]\Delta{H}_{\text{f}}^{\circ}[/latex] are known, the Born-Haber cycle can be used to determine the electron affinity of an atom.
Lattice energies calculated for ionic compounds are typically much higher than bond dissociation energies measured for covalent bonds. Whereas lattice energies typically fall in the range of 600–4000 kJ/mol (some even higher), covalent bond dissociation energies are typically between 150–400 kJ/mol for single bonds. Keep in mind, however, that these are not directly comparable values. For ionic compounds, lattice energies are associated with many interactions, as cations and anions pack together in an extended lattice. For covalent bonds, the bond dissociation energy is associated with the interaction of just two atoms.
Key Takeaways
Lattice energies are often calculated using the Born-Haber cycle, a thermochemical cycle including all of the energetic steps involved in converting elements into an ionic compound.
- Lattice energy for a solid MX: [latex]\text{MX}\left(s\right)\rightarrow{\text{M}}^{n\text{+}}\left(g\right)+{\text{X}}^{n-}\left(g\right)\Delta{H}_{\text{lattice}}[/latex]
- Lattice energy for an ionic crystal: [latex]\Delta{H}_{\text{lattice}}=\frac{\text{k}\left({\text{Q}}^{\text{+}}\right)\left({\text{Q}}^{-}\right)}{{\text{d}}}[/latex]
Exercises
- The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 200.8 pm. NaF crystallizes in the same structure as LiF but with a Na–F distance of 231 pm. Which of the following values most closely approximates the lattice energy of NaF: 510, 890, 1023, 1175, or 4090 kJ/mol? Explain your choice.
- For which of the following substances is the least energy required to convert one mole of the solid into separate ions?
- MgO
- SrO
- KF
- CsF
- MgF2
- The reaction of a metal, M, with a halogen, X2, proceeds by an exothermic reaction as indicated by this equation: [latex]\text{M}\left(s\right)+{\text{X}}_{2}\left(g\right)\rightarrow{\text{MX}}_{2}\left(s\right).[/latex] For each of the following, indicate which option will make the reaction more exothermic. Explain your answers.
- a large radius vs. a small radius for M+2
- a high ionization energy vs. a low ionization energy for M
- an increasing bond energy for the halogen
- a decreasing electron affinity for the halogen
- an increasing size of the anion formed by the halogen
- The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 201 pm. MgO crystallizes in the same structure as LiF but with a Mg–O distance of 205 pm. Which of the following values most closely approximates the lattice energy of MgO: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4090 kJ/mol? Explain your choice.
- Which compound in each of the following pairs has the larger lattice energy? Note: Mg2+ and Li+ have similar radii; O2– and F– have similar radii. Explain your choices.
- MgO or MgSe
- LiF or MgO
- Li2O or LiCl
- Li2Se or MgO
- Which compound in each of the following pairs has the larger lattice energy? Note: Ba2+ and K+ have similar radii; S2– and Cl– have similar radii. Explain your choices.
- K2O or Na2O
- K2S or BaS
- KCl or BaS
- BaS or BaCl2
- Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- MgO
- SrO
- KF
- CsF
- MgF2
- Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- K2S
- K2O
- CaS
- Cs2S
- CaO
- The lattice energy of KF is 794 kJ/mol, and the interionic distance is 269 pm. The Na–F distance in NaF, which has the same structure as KF, is 231 pm. Which of the following values is the closest approximation of the lattice energy of NaF: 682 kJ/mol, 794 kJ/mol, 924 kJ/mol, 1588 kJ/mol, or 3175 kJ/mol? Explain your answer.
Glossary
Born-Haber cycle: thermochemical cycle relating the various energetic steps involved in the formation of an ionic solid from the relevant elements
lattice energy (ΔHlattice): energy required to separate one mole of an ionic solid into its component gaseous ions