The cell membrane plays the dual roles of protecting the living cell by acting as a barrier to the outside world, yet at the same time it must allow the passage of food and waste products into and out of the cell for metabolism to proceed. How does the cell carry out these seemingly paradoxical roles? To understand this process you need to understand the makeup of the cell membrane and an important phenomenon known as diffusion.
Diffusion is the movement of a substance from an area of high concentration to an area of low concentration due to random molecular motion. All atoms and molecules possess kinetic energy, which is the energy of movement. It is this kinetic energy that makes each atom or molecule vibrate and move around. (In fact, you can quantify the kinetic energy of the atoms/molecules in a substance by measuring its temperature.) The moving atoms bounce off each other, like bumper cars in a carnival ride. The movement of particles due to this energy is called Brownian motion. As these atoms/molecules bounce off each other, the result is the movement of these particles from an area of high concentration to an area of low concentration. This is diffusion. The rate of diffusion is influenced by both temperature (how fast the particles move) and size (how big they are).

Part 1: Brownian Motion
In this part of the lab, you will use a microscope to observe Brownian motion in carmine red powder, which is a dye obtained from the pulverized guts of female cochineal beetles.
Materials
- Glass slide
- Toothpick
- Carmine red powder
- Coverslip
- Tap water
Procedure
- Obtain a microscope slide and place a drop of tap water on it.
- Using a toothpick, carefully add a very minuscule quantity of carmine red powder to the drop of water and add a coverslip.
- Observe under scanning, low, and then high power.
Lab Questions
- Describe the activity of the carmine red particles in water.
- If the slide were warmed up, would the rate of motion of the molecules speed up, slow down, or remain the same? Why?
Part 2: Diffusion across a Semipermeable Membrane
Because of its structure, the cell membrane is a semipermeable membrane. This means that SOME substances can easily diffuse through it, like oxygen, or carbon dioxide. Other substances, like glucose or sodium ions, are unable to pass through the cell membrane unless they are specifically transported via proteins embedded in the membrane itself. Whether or not a substance is able to diffuse through a cell membrane depends on the characteristics of the substance and characteristics of the membrane. In this lab, we will make dialysis tubing “cells” and explore the effect of size on a molecule’s ability to diffuse through a “cell membrane.”
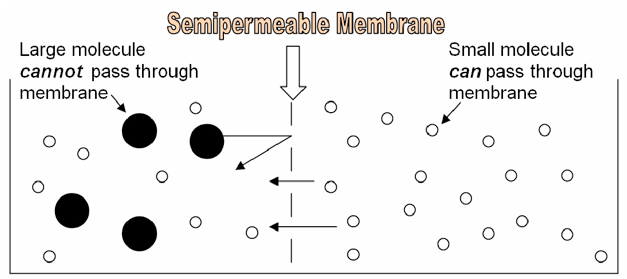
The following information might be useful in understanding and interpreting your results in this lab:
- Phenolphthalein
- Atomic formula: C20H14O4
- Atomic mass: 318.32 g/mol
- Color in acidic solution : Clear
- Color in basic solution: Pink
- Iodine
- Atomic formula: I or I2
- Materials
- Atomic mass: 126 g/mol
- Starch
- Atomic formula: (C6H10O5)n
- Atomic mass: HUGE!
- Color in Iodine: Bluish
- Sodium Hydroxide
- Atomic formula: NaOH
- Atomic mass: 40.1 g/mol
- Acid/Base: Base
Materials
- 2 pieces of dialysis tubing
- Thread
- Phenolphthalein
- Iodine
- Wax pencil
- 2 beakers
- NaOH
- Starch solution
- Pipettor
- Pipette
Procedure
- Using a wax pencil, label one beaker #1. Label the other beaker #2.
- Fill beaker #1 with 300 ml of tap water, then add 10 drops of 1 M NaOH. Do not spill the NaOH—it is very caustic!
- Fill beaker #2 with 300 ml of tap water, then add iodine drops drop by drop until the solution is bright yellow.
- Now prepare your 2 dialysis tubing “bags.” Seal one end of each dialysis tube by carefully folding the end “hotdog style” 2 times, then “hamburger style” 1 time. Tie the folded portion of the tube securely with string. It is critical that your tubing is tightly sealed, to prevent leaks.
- Add 10 ml of water and three drops of phenolphthalein to one of your dialysis tube bags. Seal the other end of the bag by carefully folding and tying as before.
- Thoroughly rinse the bag containing phenolphthalein, then place it in into the beaker containing the NaOH.
- Add 10 ml of starch solution to the other dialysis tube. Again seal the bag tightly and rinse as above. Place this bag containing the starch solution into beaker #2.
- Let diffusion occur between the bags and the solutions in the beakers.
- After 10 minutes, observe the color changes in the two bags and the external solutions. Draw a picture of each system below.

Data
Record the colors (below) and label contents inside and outside the bags (above):
| Beaker 1 | Beaker 2 | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Color inside bag | ||||
| Color outside bag (in beaker) |
||||
Lab Questions
- Which substance diffused across the membrane in beaker #1? How do you know?
- Which substance diffused across the membrane in beaker #2? How do you know?
- Why might some ions and molecules pass through the dialysis bag while others might not?
Part 3: Osmosis and the Cell Membrane
Osmosis is the movement of water across a semipermeable membrane (such as the cell membrane). The tonicity of a solution involves comparing the concentration of a cell’s cytoplasm to the concentration of its environment. Ultimately, the tonicity of a solution can be determined by examining the effect a solution has on a cell within the solution.
By definition, a hypertonic solution is one that causes a cell to shrink. Though it certainly is more complex than this, for our purposes in this class, we can assume that a hypertonic solution is more concentrated with solutes than the cytoplasm. This will cause water from the cytoplasm to leave the cell, causing the cell to shrink. If a cell shrinks when placed in a solution, then the solution is hypertonic to the cell.
If a solution is hypotonic to a cell, then the cell will swell when placed in the hypotonic solution. In this case, you can imagine that the solution is less concentrated than the cell’s cytoplasm, causing water from the solution to flow into the cell. The cell swells!
Finally, an isotonic solution is one that causes no change in the cell. You can imagine that the solution and the cell have equal concentrations, so there is no net movement of water molecules into or out of the cell.
In this exercise, you will observe osmosis by exposing a plant cell to salt water.
Prediction
What do you think will happen to the cell in this environment? Draw a picture of your hypothesis.
Materials
- Elodea leaf
- Microscope slide
- Coverslip
- 5% NaCl solution
Procedure
- Remove a leaf from an Elodea plant using the forceps.
- Make a wet mount of the leaf. Use the pond water to make your wet mount.
- Observe the Elodea cells under the compound microscope at high power (400 X) and draw a typical cell below.
- Next, add several drops of 5% salt solution to the edge of the coverslip to allow the salt to diffuse under the coverslip. Observe what happens to the cells (this may require you to search around along the edges of the leaf). Look for cells that have been visibly altered.
Results
Draw a typical cell in both pond and salt water and label the cell membrane and the cell wall.
Lab Questions
- What do you see occurring to the cell membrane when the cell was exposed to salt water? Why does this happen?
- Describe the terms hypertonic, hypotonic and isotonic.
- How would your observations change if NaCl could easily pass through the cell membrane and into the cell?
Part 4: Experimental Design
You and your group will design an experiment to determine the relative molecular weights of methylene blue and potassium permanganate. You may use a petri dish of agar, which is a jello-like medium made from a polysaccharide found in the cell walls of red algae. You will also have access to a cork borer and a small plastic ruler.
Materials
- 1 petri dish of agar
- Methlylene blue
- Potassium permanganate
- Other?
Design
Your experiment design should include all of the following portions:
- Hypothesis
- Experimental design
- Data
- Conclusions
- Further questions/other comments
