The best-known feature of skeletal muscle is its ability to contract and cause movement. Skeletal muscles act not only to produce movement but also to stop movement, such as resisting gravity to maintain posture. Small, constant adjustments of the skeletal muscles are needed to hold a body upright or balanced in any position. Muscles also prevent excess movement of the bones and joints, maintaining skeletal stability and preventing skeletal structure damage or deformation. Joints can become misaligned or dislocated entirely by pulling on the associated bones; muscles work to keep joints stable. Skeletal muscles are located throughout the body at the openings of internal tracts to control the movement of various substances. These muscles allow functions, such as swallowing, urination, and defecation, to be under voluntary control. Skeletal muscles also protect internal organs (particularly abdominal and pelvic organs) by acting as an external barrier or shield to external trauma and by supporting the weight of the organs.
Skeletal muscles contribute to the maintenance of homeostasis in the body by generating heat. Muscle contraction requires energy, and when ATP is broken down, heat is produced. This heat is very noticeable during exercise, when sustained muscle movement causes body temperature to rise, and in cases of extreme cold, when shivering produces random skeletal muscle contractions to generate heat.

Figure 1. The Three Connective Tissue Layers. Bundles of muscle fibers, called fascicles, are covered by the perimysium. Muscle fibers are covered by the endomysium.
Each skeletal muscle is an organ that consists of various integrated tissues. These tissues include the skeletal muscle fibers, blood vessels, nerve fibers, and connective tissue. Each skeletal muscle has three layers of connective tissue (called “mysia”) that enclose it and provide structure to the muscle as a whole, and also compartmentalize the muscle fibers within the muscle (Figure 1). Each muscle is wrapped in a sheath of dense, irregular connective tissue called the epimysium, which allows a muscle to contract and move powerfully while maintaining its structural integrity. The epimysium also separates muscle from other tissues and organs in the area, allowing the muscle to move independently.
Inside each skeletal muscle, muscle fibers are organized into individual bundles, each called a fascicle, by a middle layer of connective tissue called the perimysium. This fascicular organization is common in muscles of the limbs; it allows the nervous system to trigger a specific movement of a muscle by activating a subset of muscle fibers within a bundle, or fascicle of the muscle. Inside each fascicle, each muscle fiber is encased in a thin connective tissue layer of collagen and reticular fibers called the endomysium. The endomysium contains the extracellular fluid and nutrients to support the muscle fiber. These nutrients are supplied via blood to the muscle tissue.
In skeletal muscles that work with tendons to pull on bones, the collagen in the three tissue layers (the mysia) intertwines with the collagen of a tendon. At the other end of the tendon, it fuses with the periosteum coating the bone. The tension created by contraction of the muscle fibers is then transferred though the mysia, to the tendon, and then to the periosteum to pull on the bone for movement of the skeleton. In other places, the mysia may fuse with a broad, tendon-like sheet called an aponeurosis, or to fascia, the connective tissue between skin and bones. The broad sheet of connective tissue in the lower back that the latissimus dorsi muscles (the “lats”) fuse into is an example of an aponeurosis.
Every skeletal muscle is also richly supplied by blood vessels for nourishment, oxygen delivery, and waste removal. In addition, every muscle fiber in a skeletal muscle is supplied by the axon branch of a somatic motor neuron, which signals the fiber to contract. Unlike cardiac and smooth muscle, the only way to functionally contract a skeletal muscle is through signaling from the nervous system.
Skeletal Muscle Fiber Anatomy
Because skeletal muscle cells are long and cylindrical, they are commonly referred to as muscle fibers. Skeletal muscle fibers can be quite large for human cells, with diameters up to 100 μm and lengths up to 30 cm (11.8 in) in the Sartorius of the upper leg. During early development, embryonic myoblasts, each with its own nucleus, fuse with up to hundreds of other myoblasts to form the multinucleated skeletal muscle fibers. Multiple nuclei mean multiple copies of genes, permitting the production of the large amounts of proteins and enzymes needed for muscle contraction.
Some other terminology associated with muscle fibers is rooted in the Greek sarco, which means “flesh.” The plasma membrane of muscle fibers is called the sarcolemma, due to the special membrane structures found on muscle fibers, but not other cells. Likewise, the cytoplasm is referred to as sarcoplasm, and contains chemicals and organelles that are unique to muscle fibers. One chemical only found in muscle fibers is myoglobin, a protein that can bind to oxygen and store it in the muscle cell, like the hemoglobin in red blood cells. An organelle only found in muscle cells is the myofibril (Figure 2), which acts as the contractile organelle that permits the shortening of a muscle cell during a contraction. Because contraction is the primary function of a muscle cell, skeletal muscle fibers have numerous myofibrils, so many that they compose up to 80% of the sarcoplasm! In addition, skeletal muscle fibers have a specialized smooth endoplasmic reticulum that wraps around the myofibrils, called the sarcoplasmic reticulum (SR) (Figure 2). The sarcoplasmic reticulum stores calcium ions (Ca+2), and releases them into the sarcoplasmic when stimulated. Within each myofibril are the functional contractile units of a skeletal muscle fiber, called sarcomeres. Each sarcomere is composed of a highly organized arrangement of the contractile myofilaments actin (thin filament) and myosin (thick filament), along with other support proteins.

Figure 2. Muscle Fiber. A skeletal muscle fiber is surrounded by a plasma membrane called the sarcolemma, which contains sarcoplasm, the cytoplasm of muscle cells. A muscle fiber is composed of many fibrils, which give the cell its striated appearance.
For the action potential to reach the membrane of the SR, there are periodic indentations of the sarcolemma, called Transverse tubules (also known as T-tubules). You will recall that the diameter of a muscle fiber can be up to 100 μm, so these T-tubules ensure that the membrane can get close to the SR in the sarcoplasm. Where the sarcoplasmic reticulum meets the T-tubule, it forms an enlarged, dead-end swelling called a terminal cistern. The arrangement of a T-tubule with two terminal cisternae (one on either side of the T-tubule) is called a triad (Figure 5). The triad surrounds the myofibril, which contains actin and myosin.

Figure 3. The T-tubule. Narrow T-tubules permit the conduction of electrical impulses. The SR functions to regulate intracellular levels of calcium. Two terminal cisternae (where enlarged SR connects to the T-tubule) and one T-tubule comprise a triad—a “threesome” of membranes, with those of SR on two sides and the T-tubule sandwiched between them.
The Sarcomere
The striated appearance of skeletal muscle fibers is due to the arrangement of the myofilaments of actin and myosin in sequential order from one end of the muscle fiber to the other. Each packet of these microfilaments and their regulatory proteins, troponin and tropomyosin (along with other proteins) is called a sarcomere.
The sarcomere is the functional unit of the muscle fiber. The sarcomere itself is bundled within the myofibril that runs the entire length of the muscle fiber and attaches to the sarcolemma at its end. As myofibrils contract, the entire muscle cell contracts. Because myofibrils are only approximately 1.2 μm in diameter, hundreds to thousands (each with thousands of sarcomeres) can be found inside one muscle fiber. Each sarcomere is approximately 2 μm in length with a three-dimensional cylinder-like arrangement and is bordered by structures called Z-discs (also called Z-lines, because pictures are two-dimensional), to which the actin myofilaments are anchored (Figure 3). Because the actin and its troponin-tropomyosin complex (projecting from the Z-discs toward the center of the sarcomere) form strands that are thinner than the myosin, it is called the thin filament of the sarcomere. Likewise, because the myosin strands and their multiple heads (projecting from the center of the sarcomere, toward but not all to way to, the Z-discs) have more mass and are thicker, they are called the thick filament of the sarcomere.
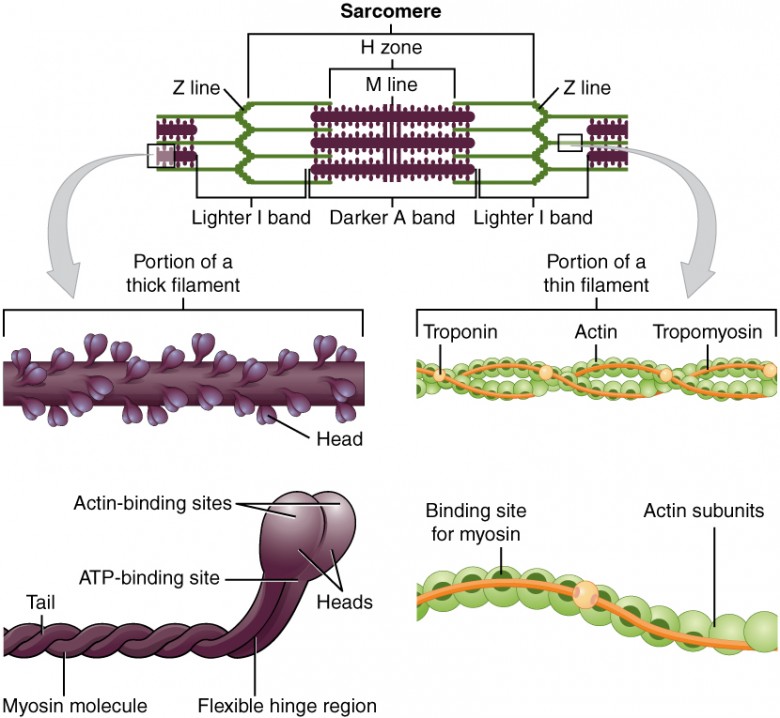
Figure 3. The Sarcomere. The sarcomere, the region from one Z-line to the next Z-line, is the functional unit of a skeletal muscle fiber.
Myosin is a protein that converts the chemical energy stored in the bonds of ATP into the kinetic energy of movement. Myosin is the force-generating protein in all muscle cells, and a coordinated effort among many myosin molecules pulling on actin, generates force for movement. Myosin molecules have two main structural parts. The tail of a myosin molecule consists of two polypeptide subunits wound together, whereas the head is composed of two globular subunits. The tail of a myosin molecule connects with other myosin molecules to form the central region of a thick filament, whereas the heads align on either end of the thick filament where thin filaments overlap with the thick filament. The point at which the head and tail of the molecule meet is flexible and allows the head to move back and forth. This allows myosin to “walk” and pull on actin filaments.
ATPase on myosin head hydrolyzes ATP to ADP. The energy released during ATP hydrolysis changes the angle of the myosin head into a “cocked” position. The myosin attaches to actin, and after releasing the ADP and Pi, the myosin head twists toward the M line, pulling actin along with it. As actin is pulled, the filaments move approximately 10 nanometers toward the M line. This movement is called the power stroke, as the thin filament “slides” over the myosin and ADP is released during this step.
When the myosin head is “cocked,” myosin is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke. At the end of the power stroke, the myosin head is in a low-energy position. Each myosin molecule may form many cross-bridges during muscle contraction. The collection of power strokes and cross-bridges allows collections of individual molecules to generate large forces.

Actin filaments are made of individual globular (spherical) protein subunits that assemble linearly into helical (twisted) filaments. Each G-actin molecule on the thin filament possesses an active site to which myosin can bind. In skeletal and cardiac muscle, this myosin binding site is covered when the muscle is relaxed, but in smooth muscle the active site on actin is always exposed.
In skeletal muscle, tropomyosin and troponin regulate contraction by controlling the interaction between actin and myosin filaments. Tropomyosin acts to block myosin binding sites on the actin filament, preventing cross-bridge formation and thus preventing contraction in a relaxed muscle. Calcium triggers muscle contraction by binding to troponin and altering its shape so that tropomyosin does not block the myosin binding sites on actin, thus allowing muscle contraction to occur. Calcium is generally an important molecule in muscle function, as we will discuss later.
Titin, as the name implies, is a very large structural protein in muscle cells. Unlike actin and myosin, which bundle together to form a multiprotein complex, titin is a single protein that holds large structures together. Thus, titin is a large, multifunctional protein (hundreds of times bigger than these other proteins) that forms an elastic filament. Titin helps align the myosin proteins and allows the muscle cell to maintain structural integrity by resisting extreme stretching, preventing damage due to overstretching.
Dystrophin is a protein that helps bind actin to the muscle cell membrane. Insufficient dystrophin production results in an inability to transfer the force of the organized actin-myosin contraction to the muscle cell membrane and ultimately to the tendons. Loss or insufficient production of this molecule causes Duchenne muscular dystrophy (DMD).
Sarcomere Anatomy—H Zone, M Line, Z Disc, I Band and A Band

Histological sections of muscle show the anatomical features of the sarcomeres. Thick filaments, composed of myosin, are visible as the A band of a sarcomere. Thin filaments, composed of actin, attach to a protein in the Z disc (or Z line) called alpha-actinin, and they are present across the entire length of the I band and a portion of the A band. The region where thick and thin filaments overlap has a dense appearance, as there is little space between the filaments. This zone where thin and thick filaments overlap is very important to muscle contraction, as it is the site where filament movement starts. Thin filaments do not extend completely into the A bands, leaving a central region of the A band that only contains thick filaments. This central region of the A band looks slightly lighter than the rest of the A band, and is called the H zone. The middle of the H zone has a vertical line called the M line, where accessory proteins hold together thick filaments.
The Neuromuscular Junction
Another specialization of the skeletal muscle is the site where a motor neuron’s terminal meets the muscle fiber—called the neuromuscular junction (NMJ). This is where the muscle fiber first responds to signaling by the motor neuron. Every skeletal muscle fiber in every skeletal muscle is innervated by a motor neuron at the NMJ. Excitation signals from the neuron are the only way to functionally activate the fiber to contract.
All living cells have membrane potentials, or electrical gradients across their membranes. The inside of the membrane is usually around -70 mV, relative to the outside. This is referred to as a cell’s resting membrane potential. Neurons and muscle cells can use their membrane potentials to generate electrical signals. They do this by controlling the movement of charged particles, called ions, across their membranes to create electrical currents. This is achieved by opening and closing specialized proteins in the membrane called ion channels. Although the currents generated by ions moving through these channel proteins are very small, they form the basis of both neural signaling and muscle contraction.
Both neurons and skeletal muscle cells are electrically excitable, meaning that they are able to generate action potentials. An action potential is a special type of depolarizing electrical signal that can travel along a cell membrane. This allows a signal to be transmitted quickly and without losing it’s strength over long distances.
In order for a skeletal muscle fiber to contract, it must receive an action potential from a somatic motor neuron. For this to occur, the electrical signal of an action potential on the somatic motor neuron is converted into a chemical signal in the form of a neurotransmitter. The neurotransmitter binds to the skeletal muscle fiber, and regenerates the action potential. This series of events occurs at the neuromuscular junction (Figure 4).

Figure 4. Motor End-Plate and Innervation. At the NMJ, the axon terminal releases ACh. The motor end-plate is the location of the ACh-receptors in the muscle fiber sarcolemma. When ACh molecules are released, they diffuse across a minute space called the synaptic cleft and bind to the receptors.
The motor neurons that tell the skeletal muscle fibers to contract originate in the spinal cord, with a smaller number located in the brainstem for activation of skeletal muscles of the face, head, and neck. These neurons have long processes, called axons, which are specialized to transmit action potentials long distances— in this case, all the way from the spinal cord to the muscle itself (which may be up to three feet away). The axons of multiple neurons bundle together to form nerves, like wires bundled together in a cable.
Signaling begins when a neuronal action potential travels along the axon of a motor neuron, and then along the individual branches to terminate at the NMJ. At the NMJ, the axon terminal releases a chemical messenger, or neurotransmitter, called acetylcholine (ACh). The ACh molecules diffuse across a minute space called the synaptic cleft and bind to ACh receptors located within the motor end-plate of the sarcolemma on the other side of the synapse. Once ACh binds, a channel in the ACh receptor opens and positively charged ions can pass through into the muscle fiber, causing it to depolarize, meaning that the membrane potential of the muscle fiber becomes less negative (closer to zero.)
As the membrane depolarizes, another set of ion channels called voltage-gated sodium channels are triggered to open. Sodium ions enter the muscle fiber, and an action potential rapidly spreads (or “fires”) along the entire membrane to initiate excitation-contraction coupling.
Things happen very quickly in the world of excitable membranes (just think about how quickly you can snap your fingers as soon as you decide to do it). Immediately following depolarization of the membrane, it repolarizes, re-establishing the negative membrane potential. Meanwhile, the ACh in the synaptic cleft is degraded by the enzyme acetylcholinesterase (AChE) so that the ACh cannot rebind to a receptor and reopen its channel, which would cause unwanted extended muscle excitation and contraction.
Propagation of an action potential along the sarcolemma is the excitation portion of excitation-contraction coupling. Recall that this excitation actually triggers the release of calcium ions (Ca+2) from its storage in the cell’s SR. The T-tubules carry the action potential into the interior of the cell, which triggers the opening of calcium channels in the membrane of the adjacent SR, causing Ca+2 to diffuse out of the SR and into the sarcoplasm. It is the arrival of Ca+2 in the sarcoplasm that initiates contraction of the muscle fiber by its contractile units, or sarcomeres.
Skeletal Myocytes—Myoblast Fusion, Myotube Organization in Skeletal Muscle Tissue and Satellite Cells


Each skeletal muscle fiber is a single skeletal muscle cell, also known as a skeletal myocyte (“myo-” refers to “muscle” and “-cyte” refers to “cell”), that is formed from the fusion of precursor cells. As described before, cell fusion leads to multinucleation of each mature muscle fiber. Each myoblast, the embryonic cell type that differentiates into muscle, contributes one nucleus when the muscle fiber is formed during development.
During development, individual myoblasts (“-blast” refers to “building” … like osteoblasts), migrate to different regions in the body and then fuse to form a myotube. A myotube is a type of syncytium, which is the term used for a group of fused cells. Skeletal muscle cells are multinucleate because the syncytium (“syn-” means “same” and “cyt” refers to “cytoplasm”) fusion retains the nucleus of each contributing myoblast. This syncytium leads to the collective sarcoplasm and sarcolemma, described above.
Mature muscle does not grow by this process. Mature cells can change in size, but new cells are not formed when muscles grow. Instead, structural proteins are added to muscle fibers in a process called hypertrophy. The reverse, when structural proteins are lost and muscle mass decreases, is called atrophy. Cellular components of muscles can also undergo changes in response to changes in muscle use.
Although the number of muscle cells is set during development, satellite cells help to repair skeletal muscle fibers. Satellite cells are similar to myoblasts in that they are able to divide, fuse and differentiate. These satellite cells are located outside the muscle fibers and are stimulated to grow and fuse with muscle cells by growth factors that are released by muscle fibers under certain forms of stress. Satellite cells can regenerate muscle fibers to a very limited extent, but they primarily help to repair damage in living cells. Satellite cells facilitate the protein synthesis required for repair and growth. If a cell is damaged to a greater extent than can be repaired by satellite cells, the muscle fibers are replaced by scar tissue in a process called fibrosis. Because scar tissue cannot contract, muscle that has sustained significant damage loses strength and cannot produce the same amount of force or endurance as it could before being damaged.
Candela Citations
- Anatomy & Physiology. Authored by: OpenStax College. Provided by: Rice University. Located at: http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@9.1. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@9.1
- Unit 6: Muscular System (Module 18). Authored by: Open Learning Initiative. Provided by: Carnegie Mellon University. Located at: https://oli.cmu.edu/jcourse/workbook/activity/page?context=43488f3780020ca60140b159062e6e31. Project: Anatomy & Physiology. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
