Australopithecus afarensis (4.2 mya)
(“southern ape” / Afar region of Ethiopia)

Figure 11.1 Forensic facial reconstruction of Australopithecus afarensis. “Australopithecus afarensis” by Cicero Moraes is licensed under CC BY-SA 3.0.
SITES
Ethiopia: Afar Depression (e.g., Hadar and Dikika)
Tanzania: Laetoli
Chad: Bahr el Ghazal
PEOPLE
Donald Johanson, Mary Leakey, Zeresenay Alemseged
INTRODUCTION
Australopithecus afarensis, or the “southern ape from Afar,” is a well-known species due to the famous “Lucy” specimen. It has been extensively studied by numerous famous paleoanthropologists. As mentioned, it is categorized as a gracile form of australopith. The species survived for over a million years in the changing East African landscape, covering a broad geographic range. The famous Laetoli footprints are attributed to Au. afarensis (see Figures 11.5 and 11.6). They provided support for the then controversial idea of habitual bipedalism, as well as the species’ presence in a more open environment.
PHYLOGENY
The most logical ancestor for Au. afarensis is Au. anamensis. The two species overlapped in time and geographic space. Some paleoanthropologists have always believed that genus: Homo is descended from Au. afarensis. Over time, others have changed their taxonomic scenarios from Au. africanus to Au. afarensis (which would formerly have been a sister lineage to Au. africanus) as our ancestor, and made Au. africanus a side branch of the robust forms. Part of the argument for classifying Au. afarensis outside of our lineage had to do with aspects of their anatomy being more derived than our own, e.g. the lateral flare of their ilia (the plural of ilium). Since the discovery of Au. sediba (Chapter 21), some scholars are back to favoring Au. africanus in our ancestry.

Figure 11.2 “Laetoli recreation.” “Laetoli recreated” by Wapondaponda is licensed under CC BY-SA 3.0.
DISCOVERY AND GEOGRAPHIC RANGE
The geographic range of Au. afarensis extends over 1,600 km from the site of Hadar in the Afar Depression of Ethiopia to the Laetoli site in Tanzania (see Figure 11.3). The holotype comes from Laetoli. There is conjecture as to whether the Ethiopian and Tanzanian material should be attributed to the same species, since the sites are distant from one another and separated in time by 800 kya. In addition, if Au. bahrelghazali is included as a geographic variant of the species, their range expands 2,500 km westward into Chad (McHenry 2015). Thus this species was very successful at exploiting a variety of environments.

Figure 11.3 Map showing the major fossil sites where specimens of Australopithecus and Paranthropus have been found. From Clement and Hillson (2013), licensed under CC BY 3.0.
With the discovery of “Lucy” (3.2 mya) (see Figure 11.7) in 1974 by Donald Johanson’s crew at the site of Hadar in the Afar Depression of Ethiopia, paleoanthropology gained momentum and the rush was on in East Africa to find more evidence of human origins. Certainly Louis and Mary Leakey recognized the importance of the Great Rift Valley, but Johanson “upped the ante” with his 3.2 mya find. In addition, since Lucy’s skeleton was almost 40% complete (making it one of the six most complete fossilized hominin skeletons older than 100 kya), much could be said about her anatomy and locomotor capabilities.
Site AL 333 at Hadar yielded remains of 13 individuals, referred to as the “First Family.” Some researchers speculated that they may have died together and thus possibly represent a social group. However, recent examination of the deposition pattern at the site suggests otherwise (see Behrensmeyer 2008).

Figure 11.4 Dikika Baby. “SelamAustralopithecus” by Jlorenz1 is licensed under CC BY-SA 3.0.
The more recently discovered “Dikika Baby” (3.3 mya) (see Figure 11.4), also known as “Selam” (meaning “peace” in the Afar language) has contributed greatly to our knowledge of development in early hominins. Dikika, meaning “nipple” in the Afar language, is the name of the nipple-shaped hill at the site of her discovery. Discovered by Zeresenay Alemseged in 2000, the three-year-old female has also been dubbed “Lucy’s Baby” due to its proximity to Hadar where Lucy was discovered. Selam is now the oldest, most complete fossil hominin. It took five years to extract the fossils from the surrounding sandstone matrix in which they were embedded. Thus we can see that not only is there difficulty in locating fossils, along with their living conditions in the desert environments of East Africa, the fossils may take years to process before all of their secrets can be revealed.
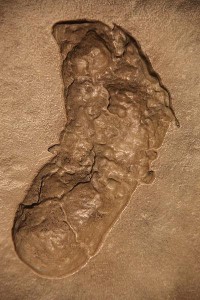
Figure 11.5 Laetoli footprint cast. “Australopithecus afarensis footprint” by Tim Evanson is licensed under CC BY-SA 2.0.
Even more recent material from the Woranso Mille site in the Afar region has some scientists questioning whether the the Au. afarensis hypodygm (all fossil material attributed to a particular species) might represent at least two species. Australopithecus deyiremeda has been suggested for the newer material.
Mary Leakey discovered the first and oldest (4.2 mya) Au. afarensis material at Laetoli, Tanzania, and the holotype (type specimen) comes from that site. Her team recovered fossil material from 23 individuals, as well as the famous Laetoli footprints. The trail of footprints extends for almost 25 m. They were made by two individuals walking side by side, with a possible third, smaller individual hopping within tracks already made by one of the adults. The prints were formed when the hominins walked through wet ash that had erupted from a nearby volcano.
PHYSICAL CHARACTERISTICS
Note: Distinguishing primitive versus derived characteristics is difficult because we do not have all body parts to compare from one species to the next. In order to determine what changed, I am considering those aspects that are more Homo-like as derived characteristics, regardless of whether predecessors possessed them. In other words, it is not perfect science!
All body parts are represented in the hypodigm, i.e. all fossils assigned to a particular species. While some debate surrounds the gait and locomotor efficiency of the species, it is fairly well accepted that they were habitual bipeds that retained some arboreal characteristics in the form of upward-oriented shoulder joints, an ape-like scapula, a high intermembral index, and curved finger bones. Their innominates and lower limbs were unquestionably those of a biped, and the big toe, while slightly divergent from the other four digits, was not nearly the grasping digit seen in apes. The buttressing of their ilium, in the form of the iliac pillar, shows that weight was being transferred through the bone in the same manner as our own. While there is evidence of the bicondylar or carrying angle of the femur, the femoral head was small and the neck (narrowing below the head) was longer in australopiths and paranthropines (i.e. robust australopiths—see Chapters 16–19), relative to Homo species. Lovejoy believes that the degree of lateral iliac flare and long femoral neck in australopiths were associated with increased leverage of the deep gluteal muscles so that they were more biomechanically efficient than modern humans (Lovejoy 1988). As we gave birth to larger-brained infants, our pelvic aperture had to expand laterally so that the femoral neck became shorter and the deep gluteal muscles became less biomechanically stable relative to australopiths. The bony elements of the Homo pelvis and hip had to become more robust to handle the increased force that the gluteal muscles generated on the bones (discussed in Lovejoy 1988 and Cartmill and Smith 2009). Based upon the Laetoli footprints, it appears that the feet of Au. afarensis were slightly inverted, which would have helped with climbing.

Figure 11.6 Replica of Laetoli footprints. “Laetoli footprints replica” by Momotarou2012 is licensed under CC BY-SA 3.0.
Au. afarensis had a prognathic, ape-like face (see Figure 11.8), a primitive skull morphology, and a small brain averaging 420 cc. They exhibited a slight sagittal crest for attachment of the temporalis muscle and a more pronounced nuchal crest, where their nuchal (posterior neck) muscles inserted on the posterior skull. The two crests were compound—a compound sagittal-nuchal crest—meaning that the sagittal crest converged at the center of the nuchal crest. Their teeth were large and their dental arcade was U-shaped, and thus more ape-like. The lower first premolar suggests a transitional phase, termed semisectorial, between the honing, sectorial (single-cusped) premolar of the apes and our more bicuspid premolars. The canines were monomorphic. Like Au. anamensis, their molars were expanded.
While their hands were capable of a precision grip, they did not have the same degree of mobility in their thumbs as later species of australopiths and paranthropines. The conical thorax is linked to climbing and a large gut, and possibly the degree of lateral flare of the iliac blades.

Figure 11.7 “Lucy.” “Lucy Mexico” by danrha is in the public domain.
The Dikika Baby (see Figure 11.4) revealed inner ear adaptations that allowed them to distinguish their head from their torso; this is important for running. Her brain was of similar size to that of a same-aged chimp. This indicates that they had a more prolonged developmental period, since the adult brain was, for the most part, larger than those of chimps. While chimps’ brains are ~380 cc, Au. afarensis’ were on average 434 cc, and ranged from 342 to 540 cc. She had both deciduous and developing permanent teeth in her jaws. Finally, her ribs were in anatomical position, which confirmed the conical thorax.

Figure 11.8 Australopithecus afarensis reconstruction. “Australopithecusafarensis reconstruction” by Durova is licensed under CC BY-SA 4.0
Au. afarensis exhibited pronounced sexual dimorphism, with males and females averaging 4´11˝ and 3´5˝ tall, respectively. Weights ranged from 64 to 99 lb. While the differences in size and morphology between the sexes might suggest that they were promiscuous and that males competed for females, their canines were monomorphic, suggesting pair-bonding. This is also supported by the change in the first mandibular premolar to being semi-sectorial.
Review of Primitive Characteristics
- High degree of sexual dimorphism.
- Low cranial capacity.
- Compound sagittal-nuchal crests:
- Slight sagittal crest.
- Pronounced nuchal crest.
- Flat cranial base.
- Prognathic jaws with U-shaped dental arcade and large ape-like incisors.
- Arboreal characteristics:
- Upward-oriented shoulder joints.
- Ape-like scapula.
- Long arms relative to legs.
- Curved finger bones.
- Conical thorax.
- Some divergence of hallux.
Review of Derived Characteristics
- Inner ear adaptations allowed for more efficient running.
- Low cusp relief and thick enamel on molars.
- Loss of honing complex:
- Semi-sectorial premolar.
- Monomorphic canines.
- Precision grip adaptation in hands.
- Bipedal hips and lower limbs.
- Prolonged juvenile dependency and brain growth.
ENVIRONMENT AND WAY OF LIFE
This species inhabited a mixed woodland environment that is thought to have been more open than previous hominin habitats. They could thus have exploited arboreal resources and moved between trees and forested areas in a fairly efficient manner. They are considered to have been scavenger-foragers, collecting wild plant foods, opportunistically hunting animals, and scavenging large game from carnivore kills. There is evidence of stone tool use at the Dikika, Ethiopia, site. Since Au. afarensis are the only known hominin from that time and location, the tool use has been attributed to them. Researchers found cut marks on bones of two large animals that were dated to 3.4 mya. Even more exciting is the recent discovery of 3.3 mya tools in association with hominin fossil material at the West Lake Turkana, Kenya, site of Lomekwi 3. While it was commonly accepted that australopiths used tools, this is the first evidence that they made them. The tools have been designated as the Lomekwian industry and have displaced the Oldowan as the earliest tool industry, preceding it by 700,000 years (Harmund et al. 2015). The tools consist of anvils, cores (stones from which flakes for cutting are removed), and flakes (see Homo habilis: “Environment and Way of Life” for more information on stone tools and their production). Like extant great apes, they also would almost certainly have used biodegradable materials for tools, such as wooden, ivory, or antler digging sticks.
Au. afarensis exhibited premolar molarization and thick molar enamel for masticating a tougher, more dry-adapted diet, such as tubers (large edible roots, e.g. yams). However, they were not yet able to grind their food as well as later hominins whose jaws could move laterally due to the reduction in canine size.
The brain of Selam shows that the juvenile dependency period was prolonged relative to chimps and hence the chimp/hominin ancestor. In addition, once infants could not hang on with their feet, mothers would have had to put their babies down periodically. Dean Falk has suggested that this pattern of mother-infant care may have led to language, in the form of what she refers to as “motherese” (Falk 2009).
It is interesting that female chimps use tools more often than do males. In addition, “woman the gatherer” should share the limelight with “man the hunter,” as women in most traditional societies collected a larger share of their family’s food. Is it possible that women invented tools? How about language? For how long have we heard about the male provisioning model for the evolution of bipedalism, “man the toolmaker,” “man the hunter,” men romancing women with the first language? Let’s stir up that cooking pot!!!

Figure 11.9 Selam reconstruction at the National Museum of Addis Ababa, Ethiopia. “Selam” by Highrey is licensed under CC BY-SA 3.0.
As mentioned, we are unsure of their mating and thus grouping pattern. Regardless of whether the First Family died together and represented a social group, Au. afarensis likely lived in groups for protection and possibly cooperation. Males were much larger than females but had lost the large canines and honing complex of Au. anamensis. Thus while the degree of sexual dimorphism was much greater than in our own species, their monomorphic teeth suggest that they were transitioning toward pair-bonding while retaining polygynous tendencies. While females may have mated polyandrously, like a fair proportion of females in our own species, it may have been in their best interest to stick with their mate for help in raising their offspring, and not jeopardizing their safety with extra-pair copulations.

Figure 11.10 Lucy by Keenan Taylor.
