Additional Exercises
-
Cycloalkanes are named based on the number of C atoms in them, just like regular alkanes, but with the prefix cyclo– on the name. What are the names of the three smallest cycloalkanes?
-
Cycloalkenes are named similarly to cycloalkanes (see Exercise 1). What are the names of the cycloalkenes with five, six, and seven C atoms?
-
Draw the bond-line structure of all noncyclic alkanes with only four C atoms.
-
Draw the bond-line structure of all noncyclic alkanes with only five C atoms.
-
Cyclic alkanes can also have substituent groups on the ring. Draw the bond-line structure of all cyclic alkanes with only four C atoms.
-
Cyclic alkanes can also have substituent groups on the ring. Draw the bond-line structure of all cyclic alkanes with only five C atoms.
-
Draw and name all possible isomers of pentene.
-
Draw and name all possible normal (that is, straight-chain) isomers of heptyne.
-
Polyunsaturated alkenes have more than one C–C double bond. Draw the carbon backbone of all possible noncyclic polyunsaturated alkenes with four C atoms and two double bonds. What are the complete molecular formulas for each possible molecule?
-
Draw the carbon backbone of all possible five-carbon cyclic alkenes with two double bonds, assuming no substituents on the ring.
-
If a hydrocarbon is combined with enough halogen, all the H atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between ethane and excess chlorine.
-
If a hydrocarbon is combined with enough halogen, all the H atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between butane and excess bromine.
-
Molecules with multiple double bonds can also participate in addition reactions. Draw the structure of the product when butadiene, CH2=CH–CH=CH2, reacts with chlorine.
-
Draw the structure of the product when allene, CH2=C=CH2, reacts with bromine.
-
What is the maximum number of methyl groups that can be on a propane backbone before the molecule cannot be named as a propane compound?
-
Explain why cycloethane cannot exist as a real molecule.
-
In the gasoline industry, what is called isooctane is actually 2,2,4-trimethylpentane. Draw the structure of isooctane.
-
Isooctane (see Exercise 17) is an isomer of what straight-chain alkane?
-
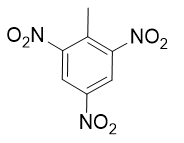
Phenol is hydroxybenzene, the simplest aromatic alcohol. Picric acid is an explosive derivative of phenol whose formal name is 2,4,6-trinitrophenol. With reference to Exercise 19, draw the structure of picric acid.
-
Draw the structures of all possible straight-chain isomers of bromopentane.
-
Draw the structures of all the possible isomers of butanol. Include branched isomers.
-
What is the final product of the double elimination of HCl from 1,1-dichloroethane?
-
Draw the structure of the final product of the double elimination of 1,3-dibromopropane.
-
Draw the structure of and name the alcohol whose double elimination would yield the same product as in Exercise 23. Name the molecule as a hydroxyl-substituted compound.
-
Draw the structure of and name the alcohol whose double elimination would yield the same product as in Exercise 24. Name the molecule as a hydroxyl-substituted compound.
-
Draw the smallest molecule that can have a separate aldehyde and carboxylic acid group.
-
Ethyl acetate is a common ingredient in nail-polish remover because it is a good solvent. Draw the structure of ethyl acetate.
-
A lactone is an ester that has its ester functional group in a ring. Draw the structure of the smallest possible lactone. (It is called acetolactone, which might give you a hint about its structure.)
-
Draw the structure of diethyl ether, once used as an anesthetic.
-
The smallest cyclic ether is called an epoxide. Draw its structure.
-
Write the chemical reaction of HCl with trimethylamine.
-
Putrescine and cadaverine are molecules with two amine groups on the opposite ends of a butane backbone and a pentane backbone, respectively. They are both emitted by rotting corpses. Draw their structures and determine their molecular formulas.
-
With four monomers, draw two possible structures of a copolymer composed of ethylene and propylene.
-
With four monomers, draw two possible structures of a copolymer composed of ethylene and styrene.
-
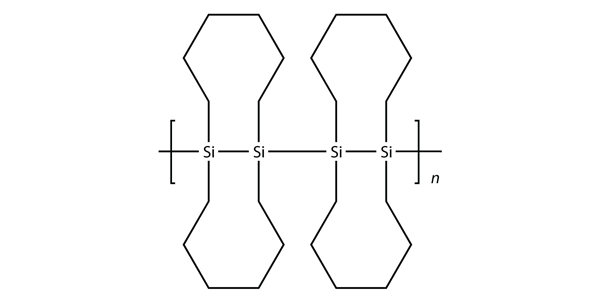
One of the ingredients in the original Silly Putty was a silicone polymer with two methyl groups on each Si atom. Draw this silicone.
Answers
1. cyclopropane, cyclobutane, and cyclopentane
5.
7.
9.
Both molecular formulas are C4H6.
11. C2H6 + 6 Cl2 → C2Cl6 + 6 HCl
13.
15. two
17.
19.
21.
23. ethyne
25. The names are 1,2-dihydroxyethane and 1,1-dihydroxyethane, respectively.
29.
31.
33. (CH3)3N + HCl → (CH3)3NHCl
35. (answers will vary)
37.
Candela Citations
- Authored by: David W. Ball and Jessie A. Key. Located at: https://opentextbc.ca/introductorychemistry/. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike