Objectives
After completing this section, you should be able to
- write the equation for the epoxidation of an alkene using meta-chloroperoxybenzoic acid.
- identify the alkene, reagents, or both, that must be used to prepare a given epoxide.
- write the equation for the hydroxylation of an alkene using osmium tetroxide, and draw the structure of the cyclic intermediate.
- draw the structure of the diol formed from the reaction of a given alkene with osmium tetroxide.
- identify the alkene, the reagents, or both, that must be used to prepare a given 1,2-diol.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- diol
- glycol
- hydroxylation
Oxacyclopropane rings, also called epoxide rings, are useful reagents that may be opened by further reaction to form anti vicinal diols. One way to synthesize oxacyclopropane rings is through the reaction of an alkene with peroxycarboxylic acid.
Oxacyclopropane Synthesis by Peroxycarboxylic Acid
Oxacyclopropane synthesis by peroxycarboxylic acid requires an alkene and a peroxycarboxylic acid as well as an appropriate solvent. The peroxycarboxylic acid has the unique property of having an electropositive oxygen atom on the COOH group. The reaction is initiated by the electrophilic oxygen atom reacting with the nucleophilic carbon-carbon double bond. The mechanism involves a concerted reaction with a four-part, circular transition state. The result is that the originally electropositive oxygen atom ends up in the oxacyclopropane ring and the COOH group becomes COH.
Mechanism
Peroxycarboxylic acids are generally unstable. An exception is meta-chloroperoxybenzoic acid, shown in the mechanism above. Often abbreviated MCPBA, it is a stable crystalline solid. Consequently, MCPBA is popular for laboratory use. However, MCPBA can be explosive under some conditions.

Peroxycarboxylic acids are sometimes replaced in industrial applications by monoperphthalic acid, or the monoperoxyphthalate ion bound to magnesium, which gives magnesium monoperoxyphthalate (MMPP). In either case, a nonaqueous solvent such as chloroform, ether, acetone, or dioxane is used. This is because in an aqueous medium with any acid or base catalyst present, the epoxide ring is hydrolyzed to form a vicinal diol, a molecule with two OH groups on neighboring carbons. (For more explanation of how this reaction leads to vicinal diols, see below.) However, in a nonaqueous solvent, the hydrolysis is prevented and the epoxide ring can be isolated as the product. Reaction yields from this reaction are usually about 75%. The reaction rate is affected by the nature of the alkene, with more nucleophilic double bonds resulting in faster reactions.
Example

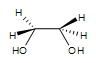
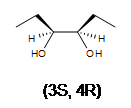
Since the transfer of oxygen is to the same side of the double bond, the resulting oxacyclopropane ring will have the same stereochemistry as the starting alkene. A good way to think of this is that the alkene is rotated so that some constituents are coming forward and some are behind. Then, the oxygen is inserted on top. (See the product of the above reaction.) One way the epoxide ring can be opened is by an acid catalyzed oxidation-hydrolysis. Oxidation-hydrolysis gives a vicinal diol, a molecule with OH groups on neighboring carbons. For this reaction, the dihydroxylation is anti since, due to steric hindrance, the ring is attacked from the side opposite the existing oxygen atom. Thus, if the starting alkene is trans, the resulting vicinal diol will have one S and one R stereocenter. But, if the starting alkene is cis, the resulting vicinal diol will have a racemic mixture of S, S and R, R enantiomers.
Examples
Problems
1. Predict the product of the reaction of cis-2-hexene with MCPBA (meta-chloroperoxybenzoic acid)
a) in acetone solvent.

b) in an aqueous medium with acid or base catalyst present.

2. Predict the product of the reaction of trans-2-pentene with magnesium monoperoxyphthalate (MMPP) in a chloroform solvent.

3. Predict the product of the reaction of trans-3-hexene with MCPBA in ether solvent.

4. Predict the reaction of propene with MCPBA.
a) in acetone solvent

b) after aqueous work-up.

5. Predict the reaction of cis-2-butene in chloroform solvent.

Anti Dihydroxylation
Epoxides may be cleaved by aqueous acid to give glycols that are often diastereomeric with those prepared by the syn-hydroxylation reaction described above. Proton transfer from the acid catalyst generates the conjugate acid of the epoxide, which is attacked by nucleophiles such as water in the same way that the cyclic bromonium ion described above undergoes reaction. The result is anti-hydroxylation of the double bond, in contrast to the syn-stereoselectivity of the earlier method. In the following equation this procedure is illustrated for a cis-disubstituted epoxide, which, of course, could be prepared from the corresponding cis-alkene. This hydration of an epoxide does not change the oxidation state of any atoms or groups.

Syn Dihydroxylation
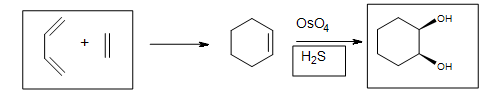
Osmium tetroxide oxidizes alkenes to give glycols through syn addition. A glycol, also known as a vicinal diol, is a compound with two -OH groups on adjacent carbons.
Introduction
The reaction with $$OsO_4$$ is a concerted process that has a cyclic intermediate and no rearrangements. Vicinal syn dihydroxylation complements the epoxide-hydrolysis sequence which constitutes an anti dihydroxylation of an alkene. When an alkene reacts with osmium tetroxide, stereocenters can form in the glycol product. Cis alkenes give meso products and trans alkenes give racemic mixtures.
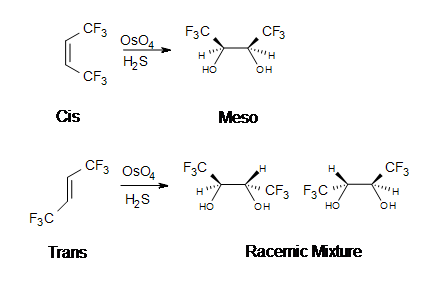
$$OsO_4$$ is formed slowly when osmium powder reacts with gasoues $$O_2$$ at ambient temperature. Reaction of bulk solid requires heating to 400 °C:
\[Os_{(s)} + 2O_{2\;(g)} \rightarrow OS_4\]
Since Osmium tetroxide is expensive and highly toxic, the reaction with alkenes has been modified. Catalytic amounts of OsO4 and stoichiometric amounts of an oxidizing agent such as hydrogen peroxide are now used to eliminate some hazards. Also, an older reagent that was used instead of OsO4 was potassium permanganate, $$KMnO_4$$. Although syn diols will result from the reaction of KMnO4 and an alkene, potassium permanganate is less useful since it gives poor yields of the product because of overoxidation.
Mechanism
- Electrophilic attack on the alkene
- Pi bond of the alkene acts as the nucleophile and reacts with osmium (VIII) tetroxide (OsO4)
- 2 electrons from the double bond flows toward the osmium metal
- In the process, 3 electron pairs move simultaneously
- Cyclic ester with Os (VI) is produced
- Reduction
- H2S reduces the cyclic ester
- NaHSO4 with H2O may be used
- Forms the syn-1,2-diol (glycol)
- H2S reduces the cyclic ester
Example: Dihydroxylation of 1-ethyl-1-cycloheptene
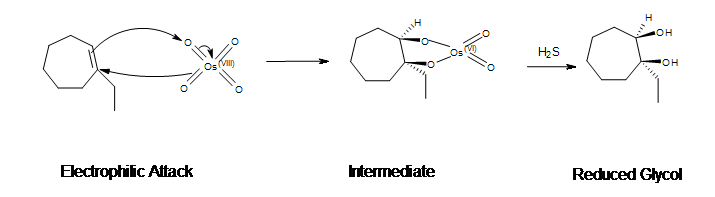
Hydroxylation of alkenes
Dihydroxylated products (glycols) are obtained by reaction with aqueous potassium permanganate (pH > 8) or osmium tetroxide in pyridine solution. Both reactions appear to proceed by the same mechanism (shown below); the metallocyclic intermediate may be isolated in the osmium reaction. In basic solution the purple permanganate anion is reduced to the green manganate ion, providing a nice color test for the double bond functional group. From the mechanism shown here we would expect syn-stereoselectivity in the bonding to oxygen, and regioselectivity is not an issue.

When viewed in context with the previously discussed addition reactions, the hydroxylation reaction might seem implausible. Permanganate and osmium tetroxide have similar configurations, in which the metal atom occupies the center of a tetrahedral grouping of negatively charged oxygen atoms. How, then, would such a species interact with the nucleophilic pi-electrons of a double bond? A possible explanation is that an empty d-orbital of the electrophilic metal atom extends well beyond the surrounding oxygen atoms and initiates electron transfer from the double bond to the metal, in much the same fashion noted above for platinum. Back-bonding of the nucleophilic oxygens to the antibonding π*-orbital completes this interaction. The result is formation of a metallocyclic intermediate, as shown above.
Chemical Highlight
Antitumor drugs have been formed by using dihydroxylation. This method has been applied to the enantioselective synthesis of ovalicin, which is a class of fungal-derived products called antiangiogenesis agents. These antitumor products can cut off the blood supply to solid tumors. A derivative of ovalicin, TNP-470, is chemically stable, nontoxic, and noninflammatory. TNP-470 has been used in research to determine its effectiveness in treating cancer of the breast, brain, cervix, liver, and prostate.
Outside links
References
- Dehestani, Ahmad et al. (2005). Ligand-assisted reduction of osmium tetroxide with molecular hydrogen via a [3+2] mechanism. Journal of the American Chemical Society, 2005, 127 (10), 3423-3432.
- Sorrell, Thomas, N. Organic Chemistry. New York: University Science Books, 2006.
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th Edition. New York: W. H. Freeman & Company, 2007.
Examples
Problems
Questions:
1. Give the major product.
.bmp?revision=1&size=bestfit&width=131&height=51#fixme)
2. What is the product in the dihydroxylation of (Z)-3-hexene?

3. What is the product in the dihydroxylation of (E)-3-hexene?
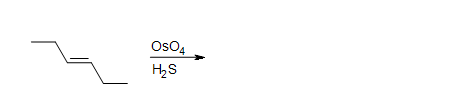
4. Draw the intermediate of this reaction.
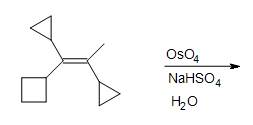
5. Fill in the missing reactants, reagents, and product.
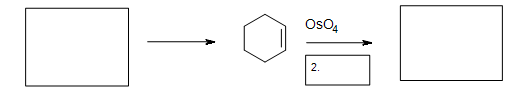
Solutions
1.
2.
3.
4.
5.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Shivam Nand
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Kristen Perano