Objectives
After completing this section, you should be able to
- determine the degree of unsaturation of an organic compound, given its molecular formula, and hence determine the number of double bonds, triple bonds and rings present in the compound.
- draw all the possible isomers that correspond to a given molecular formula containing only carbon (up to a maximum of six atoms) and hydrogen.
- draw a specified number of isomers that correspond to a given molecular formula containing carbon, hydrogen, and possibly other elements, such as oxygen, nitrogen and the halogens.
Key TERMS
Make certain that you can define, and use in context, the key terms below.
- degree of unsaturation
- saturated
- unsaturated
There are many ways one can go about determining the structure of an unknown organic molecule. Although, nuclear magnetic resonance (NMR) and infrared radiation (IR) are the primary ways of determining molecular structures, calculating the degrees of unsaturation is useful information since knowing the degrees of unsaturation make it easier for one to figure out the molecular structure; it helps one double-check the number of $$\pi$$ bonds and/or cyclic rings.
Saturated and Unsaturated Molecules
In the lab, saturation may be thought of as the point when a solution cannot dissolve anymore of a substance added to it. In terms of degrees of unsaturation, a molecule only containing single bonds with no rings is considered saturated.
| CH3CH2CH3 | 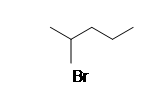 |
.bmp?revision=1&size=bestfit&width=113&height=128#fixme) |
1-methyoxypentane |
Unlike saturated molecules, unsaturated molecules contain double bond(s), triple bond(s) and/or ring(s).
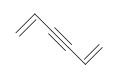
| CH3CH=CHCH3 | 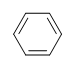 |
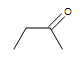 |
 |
3-chloro-5-octyne |
Calculating The Degree of Unsaturation (DoU)
If the molecular formula is given, plug in the numbers into this formula:
\[ DoU= \dfrac{2C+2+N-X-H}{2} \tag{7.2.1}\]
- C is the number of carbons
- N is the number of nitrogens
- X is the number of halogens (F, Cl, Br, I)
- H is the number of hydrogens
As stated before, a saturated molecule contains only single bonds and no rings. Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms possible to be an acyclic alkane. Thus, the number of hydrogens can be represented by 2C+2, which is the general molecular representation of an alkane. As an example, for the molecular formula C3H4 the number of actual hydrogens needed for the compound to be saturated is 8 [2C+2=(2×3)+2=8]. The compound needs 4 more hydrogens in order to be fully saturated (expected number of hydrogens-observed number of hydrogens=8-4=4). Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as saturated. Hence, the DoB formula divides by 2. The formula subtracts the number of X’s because a halogen (X) replaces a hydrogen in a compound. For instance, in chloroethane, C2H5Cl, there is one less hydrogen compared to ethane, C2H6.
For a compound to be saturated, there is one more hydrogen in a molecule when nitrogen is present. Therefore, we add the number of nitrogens (N). This can be seen with C3H9N compared to C3H8. Oxygen and sulfur are not included in the formula because saturation is unaffected by these elements. As seen in alcohols, the same number of hydrogens in ethanol, C2H5OH, matches the number of hydrogens in ethane, C2H6.
The following chart illustrates the possible combinations of the number of double bond(s), triple bond(s), and/or ring(s) for a given degree of unsaturation. Each row corresponds to a different combination.
- One degree of unsaturation is equivalent to 1 ring or 1 double bond (1 $$ \pi $$ bond).
- Two degrees of unsaturation is equivalent to 2 double bonds, 1 ring and 1 double bond, 2 rings, or 1 triple bond (2 $$ \pi $$ bonds).
|
DoU
|
Possible combinations of rings/ bonds
|
||
|
# of rings
|
# of double bonds
|
# of triple bonds
|
|
|
1
|
1
|
0
|
0
|
|
0
|
1
|
0
|
|
|
2
|
2
|
0
|
0
|
|
0
|
2
|
0
|
|
|
0
|
0
|
1
|
|
|
1
|
1
|
0
|
|
Remember, the degrees of unsaturation only gives the sum of double bonds, triple bonds and/or rings. For instance, a degree of unsaturation of 3 can contain 3 rings, 2 rings+1 double bond, 1 ring+2 double bonds, 1 ring+1 triple bond, 1 double bond+1 triple bond, or 3 double bonds.
ExampLE
Benzene
What is the Degree of Unsaturation for Benzene?
SOLUTION
References
- Vollhardt, K. P.C. & Shore, N. (2007). Organic Chemistry (5thEd.). New York: W. H. Freeman. (473-474)
- Shore, N. (2007). Study Guide and Solutions Manual for Organic Chemistry (5th Ed.). New York: W.H. Freeman. (201)
Examples
Problems
- Are the following molecules saturated or unsaturated:
-
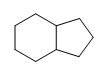 (b.)
(b.)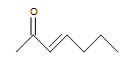 (c.)
(c.) 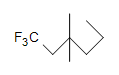 (d.) C10H6N4
(d.) C10H6N4
-
- Using the molecules from 1., give the degrees of unsaturation for each.
- Calculate the degrees of unsaturation for the following molecular formulas:
- (a.) C9H20 (b.) C7H8 (c.) C5H7Cl (d.) C9H9NO4
- Using the molecular formulas from 3, are the molecules unsaturated or saturated.
- Using the molecular formulas from 3, if the molecules are saturated, how many rings/double bonds/triple bonds are predicted?
- (d.) unsaturated5.(a.) 0 (Remember-a saturated molecule only contains single bonds)
(b.) The molecule can contain any of these combinations (i) 4 double bonds (ii) 4 rings (iii) 2 double bonds+2 rings (iv) 1 double bond+3 rings (v) 3 double bonds+1 ring (vi) 1 triple bond+2 rings (vii) 2 triple bonds (viii) 1 triple bond+1 double bond+1 ring (ix) 1 triple bond+2 double bonds
(c.) (i) 1 triple bond (ii) 1 ring+1 double bond (iii) 2 rings (iv) 2 double bonds
(d.) (i) 3 triple bonds (ii) 2 triple bonds+2 double bonds (iii) 2 triple bonds+1 double bond+1 ring (iv)… (As you can see, the degrees of unsaturation only gives the sum of double bonds, triple bonds and/or ring. Thus, the formula may give numerous possible structures for a given molecular formula.)
Answers
1.
2. If the molecular structure is given, the easiest way to solve is to count the number of double bonds, triple bonds and/or rings. However, you can also determine the molecular formula and solve for the degrees of unsaturation by using the formula.
3. Use the formula to solve
4.
Exercises
Questions
1.
Calculate degrees of unsaturation (DoU) for the following, and propose a structure for each.
A – C5H8
B – C4H4
2.
Calculate the degree of unsaturation (DoU) for the following molecules
A – C5H5N
B – C5H5NO2
C – C5H5Br
3.
The following molecule is caffeine (C8H10N4O2), determine the degrees of unsaturation (DoU).

Solutions
1.
2.
3.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Kim Quach (UCD)