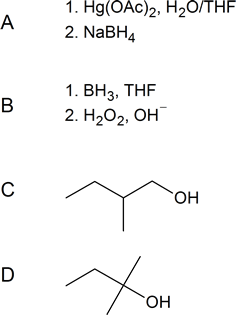Objectives
After completing this section, you should be able to
- identify hydroboration (followed by oxidation) as a method for bringing about the (apparently) non-Markovnikov addition of water to an alkene.
- write an equation for the formation of a trialkylborane from an alkene and borane.
- write an equation for the oxidation of a trialkylborane to an alcohol.
- draw the structure of the alcohol produced by the hydroboration, and subsequent oxidation, of a given alkene.
- determine whether a given alcohol should be prepared by oxymercuration-demercuration or by hydroboration-oxidation, and identify the alkene and reagents required to carry out such a synthesis.
- write the detailed mechanism for the addition of borane to an alkene, and explain the stereochemistry and regiochemistry of the reaction.
Key Terms
Make certain that you can define, and use in context, the key term below.
- hydroboration
Study Notes
The two most important factors influencing organic reactions are polar (or electronic) effects and steric effects.
Hydroboration-Oxidation is a two step pathway used to produce alcohols. The reaction proceeds in an Anti-Markovnikov manner, where the hydrogen (from BH3 or BHR2) attaches to the more substituted carbon and the boron attaches to the least substituted carbon in the alkene bouble bond. Furthermore, the borane acts as a lewis acid by accepting two electrons in its empty p orbital from an alkene that is electron rich. This process allows boron to have an electron octet. A very interesting characteristic of this process is that it does not require any activation by a catalyst. The Hydroboration mechanism has the elements of both hydrogenation and electrophilic addition and it is a stereospecific (syn addition), meaning that the hydroboration takes place on the same face of the double bond, this leads cis stereochemistry.
Introduction
Hydroboration-oxidation of alkenes has been a very valuable laboratory method for the stereoselectivity and regioselectivity of alkenes. An Additional feature of this reaction is that it occurs without rearrangement.
The Borane Complex
First off it is very imporatnt to understand little bit about the structure and the properties of the borane molecule. Borane exists naturally as a very toxic gas and it exists as dimer of the general formula B2H6 (diborane). Additionally, the dimer B2H6ignites spontaneously in air. Borane is commercially available in ether and tetrahydrofuran (THF), in these solutions the borane can exist as a lewis acid-base complex, which allows boron to have an electron octet.BH3→B2H6

The Mechanism
Step #1
- Part #1: Hydroboration of the alkene. In this first step the addittion of the borane to the alkene is initiated and prceeds as a concerted reaction because bond breaking and bond formation occurs at the same time. This part consists of the vacant 2p orbital of the boron electrophile pairing with the electron pair of the ? bondof the nucleophile.

Transition state
.bmp?revision=1&size=bestfit&width=441&height=242#fixme)
* Note that a carbocation is not formed. Therefore, no rearrangement takes place.
- Part #2: The Anti Markovnikov addition of Boron. The boron adds to the less substituted carbon of the alkene, which then places the hydrogen on the more substituted carbon. Both, the boron and the hydrogen add simultaneously on the same face of the double bond (syn addition).

Oxidation of the Trialkylborane by Hydrogen Peroxide
Step #2
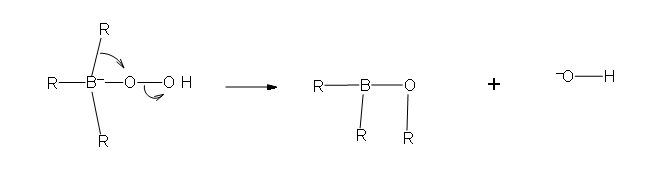
- Part #1: the first part of this mechanism deals with the donation of a pair of electrons from the hydrogen peroxide ion. the hydrogen peroxide is the nucleophile in this reaction because it is the electron donor to the newly formed trialkylborane that resulted from hydroboration.

.bmp?revision=1&size=bestfit&width=696&height=239#fixme)
- Part 2: In this second part of the mechanism, a rearrangement of an R group with its pair of bonding electrons to an adjacent oxygen results in the removal of a hydroxide ion.
Two more of these reactions with hydroperoxide will occur in order give a trialkylborate
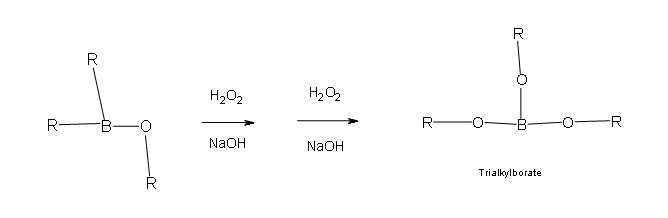
- Part 3: This is the final part of the Oxidation process. In this part the trialkylborate reacts with aqueous NaOH to give the alcohol and sodium borate.

If you need additional visuals to aid you in understanding the mechanism, click on the outside links provided here that will take you to other pages and media that are very helpful as well.
Stereochemistry of hydroboration
The hydroboration reaction is among the few simple addition reactions that proceed cleanly in a syn fashion. As noted above, this is a single-step reaction. Since the bonding of the double bond carbons to boron and hydrogen is concerted, it follows that the geometry of this addition must be syn. Furthermore, rearrangements are unlikely inasmuch as a discrete carbocation intermediate is never formed. These features are illustrated for the hydroboration of α-pinene.

Since the hydroboration procedure is most commonly used to hydrate alkenes in an anti-Markovnikov fashion, we also need to know the stereoselectivity of the second oxidation reaction, which substitutes a hydroxyl group for the boron atom. Independent study has shown this reaction takes place with retention of configuration so the overall addition of water is also syn.
The hydroboration of α-pinene also provides a nice example of steric hindrance control in a chemical reaction. In the less complex alkenes used in earlier examples the plane of the double bond was often a plane of symmetry, and addition reagents could approach with equal ease from either side. In this case, one of the methyl groups bonded to C-6 (colored blue in the equation) covers one face of the double bond, blocking any approach from that side. All reagents that add to this double bond must therefore approach from the side opposite this methyl.
References
- Vollhardt, Peter, and Neil Shore. Organic Chemistry: Structure and Function. 5th. New York: W.H. Freeman and Company, 2007.
- Foote, S. Christopher, and William H. Brown. Organic Chemistry. 5th. Belmont, CA: Brooks/Cole Cengage Learning, 2005.
- Bruice, Paula Yurkanis. Oragnic Chemistry. 5th. CA. Prentice Hall, 2006.
- Bergbreiter E. David , and David P. Rainville. Stereochemistry of hydroboration-oxidation of terminal alkenes. J. Org. Chem., 1976, 41 (18), pp 3031–3033
- Ilich, Predrag-Peter; Rickertsen, Lucas S., and Becker Erienne. Polar Addition to C=C Group: Why Is Anti-Markovnikov Hydroboration-Oxidation of Alkenes Not “Anti-“? Journal of Chemical Education., 2006, v83, n11, pg 1681-1685
Examples
Problems
What are the products of these following reactions?
#1.

#2.

#3.
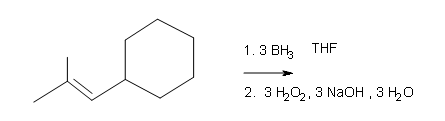
Draw the structural formulas for the alcohols that result from hydroboration-oxidation of the alkenes shown.
#4.
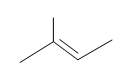
#5. (E)-3-methyl-2-pentene
If you need clarification or a reminder on the nomenclature of alkenes refer to the link below on naming the alkenes.
Answers
Exercises
Question
- Write out the reagents or products (A–D) shown in the following reaction schemes.
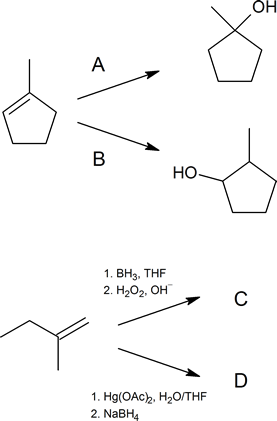
Solution
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Jim Clark (Chemguide.co.uk)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
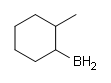 #2.
#2. #3.
#3.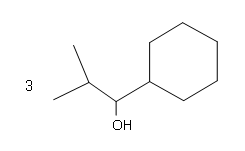 #4.
#4.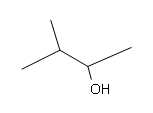 #5.
#5.