Objectives
After completing this section, you should be able to
- provide the correct IUPAC name for an acyclic or cyclic alkene, given its Kekulé, condensed or shorthand structure.
- draw the Kekulé, condensed or shorthand structure of an alkene (cyclic or acyclic), given its IUPAC name.
- give the IUPAC equivalent of the following trivial names: ethylene, propylene, isobutylene and isoprene.
- draw the structure of a vinyl (ethenyl) and allyl (2-propenyl) group, and use these names in alkene nomenclature.
Study Notes
This course uses IUPAC nomenclature; therefore, you need not usually memorize a large number of trivial names. However, you will encounter some trivial names so frequently in books and articles that they soon become familiar.
An alkene that can exhibit geometric isomerism has not been properly named unless its name specifies whether the double bond (or bonds) is (or are) cis or trans. The most effective way of giving this information is discussed, and more details of cis and trans follow in Section 7.4.
Alkenes contain carbon-carbon double bonds and are unsaturated hydrocarbons with the molecular formula is CnH2n. This is also the same molecular formula as cycloalkanes. Alkenes are named by dropping the -ane ending of the parent and adding -ene.
Introduction
The parent structure is the longest chain containing both carbon atoms of the double bond. The two carbon atoms of a double bond and the four atoms attached to them lie in a plane, with bond angles of approximately 120° A double bond consists of one sigma bond formed by overlap of sp2hybrid orbitals and one pi bond formed by overlap of parallel 2 p orbitals

The Basic Rules
For straight chain alkenes, it is the same basic rules as nomenclature of alkanes except change the suffix to “-ene.”
- Find the Longest Carbon Chain that Contains the Carbon Carbon double bond. If you have two ties for longest Carbon chain, and both chains contain a Carbon Carbon double bond, then identify the most substituted chain.
- Give the lowest possible number to the Carbon Carbon double bond.
- Do not need to number cycloalkenes because it is understood that the double bond is in the one position.
- Alkenes that have the same molecular formula but the location of the doble bonds are different means they are constitutional isomers.
- Functional Groups with higher priority:
- Add substituents and their position to the alkene as prefixes. Of course remember to give the lowest numbers possible. And remember to name them in alphabetical order when writting them.
- Next is identifying stereoisomers. when there are only two non hydrogen attachments to the alkene then use cis and trans to name the molecule.
In this diagram this is a cis conformation. It has both the substituents going upward. This molecule would be called (cis) 5-chloro-3-heptene.)
Trans would look like this
v. On the other hand if there are 3 or 4 non-hydrogen different atoms attached to the alkene then use the E, Z system.
E (entgegen) means the higher priority groups are opposite one another relative to the double bond.
Z (zusammen) means the higher priority groups are on the same side relative to the double bond.
(You could think of Z as Zame Zide to help memorize it.)
In this example it is E-4-chloro-3-heptene. It is E because the Chlorine and the CH2CH3 are the two higher priorities and they are on opposite sides.
vi. A hydroxyl group gets precedence over th double bond. Therefore alkenes containing alchol groups are called alkenols. And the prefix becomes –enol. And this means that now the alcohol gets lowest priority over the alkene.
vii. Lastly remember that alkene substituents are called alkenyl. Suffix –enyl.
Here is a chart containing the systemic name for the first twenty straight chain alkenes.
| Name | Molecular formula |
| Ethene | C2H4 |
| Propene | C3H6 |
| Butene | C4H8 |
| Pentene | C5H10 |
| Hexene | C6H12 |
| Heptene | C7H14 |
| Octene | C8H16 |
| Nonene | C9H18 |
| Decene | C10H20 |
| Undecene | C11H22 |
| Dodecene | C12H24 |
| Tridecene | C13H26 |
| Tetradecene | C14H28 |
| Pentadecene | C15H30 |
| Hexadecene | C16H32 |
| Heptadecene | C17H34 |
| Octadecene | C18H36 |
| Nonadecene | C19H38 |
| Eicosene | C20H40 |
Did you notice how there is no methene? Because it is impossible for a Carbon to have a double bond with nothing.
Geometric Isomers
Double bonds can exist as geometric isomers and these isomers are designated by using either the cis / trans designation or the modern E / Z designation.
cis Isomers
.The two largest groups are on the same side of the double bond.

trans Isomers
…The two largest groups are on opposite sides of the double bond.

E/Z nomenclature
E = entgegan (“trans”) Z = zusamen (“cis”)
Priority of groups is based on the atomic mass of attached atoms (not the size of the group). An atom attached by a multiple bond is counted once for each bond.
fluorine atom > isopropyl group > n-hexyl group
deuterium atom > hydrogen atom
-CH2-CH=CH2 > -CH2CH2CH3
Example
Try to name the following compounds using both conventions…


Common names
Remove the -ane suffix and add -ylene. There are a couple of unique ones like ethenyl’s common name is vinyl and 2-propenyl’s common name is allyl. That you should know are…
- vinyl substituent H2C=CH-
- allyl substituent H2C=CH-CH2–
- allene molecule H2C=C=CH2
- isoprene

Endocyclic Alkenes
Endocyclic double bonds have both carbons in the ring and exocyclic double bonds have only one carbon as part of the ring.

Cyclopentene is an example of an endocyclic double bond.

Methylenecylopentane is an example of an exocyclic double bond.

Name the following compounds…

1-methylcyclobutene. The methyl group places the double bond. It is correct to also name this compound as 1-methylcyclobut-1-ene.

1-ethenylcyclohexene, the methyl group places the double bond. It is correct to also name this compound as 1-ethenylcyclohex-1-ene. A common name would be 1-vinylcyclohexene.
Try to draw structures for the following compounds…
- 2-vinyl-1,3-cyclohexadiene
Examples

Both these compounds have double bonds, making them alkenes. In example (1) the longest chain consists of six carbons, so the root name of this compound will be hexene. Three methyl substituents (colored red) are present. Numbering the six-carbon chain begins at the end nearest the double bond (the left end), so the methyl groups are located on carbons 2 & 5. The IUPAC name is therefore: 2,5,5-trimethyl-2-hexene.
In example (2) the longest chain incorporating both carbon atoms of the double bond has a length of five. There is a seven-carbon chain, but it contains only one of the double bond carbon atoms. Consequently, the root name of this compound will be pentene. There is a propyl substituent on the inside double bond carbon atom (#2), so the IUPAC name is: 2-propyl-1-pentene.

The double bond in example (3) is located in the center of a six-carbon chain. The double bond would therefore have a locator number of 3 regardless of the end chosen to begin numbering. The right hand end is selected because it gives the lowest first-substituent number (2 for the methyl as compared with 3 for the ethyl if numbering were started from the left). The IUPAC name is assigned as shown.
Example (4) is a diene (two double bonds). Both double bonds must be contained in the longest chain, which is therefore five- rather than six-carbons in length. The second and fourth carbons of this 1,4-pentadiene are both substituted, so the numbering begins at the end nearest the alphabetically first-cited substituent (the ethyl group).

These examples include rings of carbon atoms as well as some carbon-carbon triple bonds. Example (6) is best named as an alkyne bearing a cyclobutyl substituent. Example (7) is simply a ten-membered ring containing both a double and a triple bond. The double bond is cited first in the IUPAC name, so numbering begins with those two carbons in the direction that gives the triple bond carbons the lowest locator numbers. Because of the linear geometry of a triple bond, a-ten membered ring is the smallest ring in which this functional group is easily accommodated. Example (8) is a cyclooctatriene (three double bonds in an eight-membered ring). The numbering must begin with one of the end carbons of the conjugated diene moiety (adjacent double bonds), because in this way the double bond carbon atoms are assigned the smallest possible locator numbers (1, 2, 3, 4, 6 & 7). Of the two ways in which this can be done, we choose the one that gives the vinyl substituent the lower number.
Outside links
References
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th Edition. New York: W. H. Freeman & Company, 2007.
Examples
Problems
1. Try to name the following compounds…


2. Try to draw structures for the following compounds…
- 2-pentene
- 3-heptene
3. Give the double bond the lowest possible numbers regardless of substituent placement.
• Try to name the following compound…
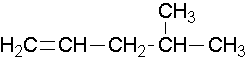
• Try to draw a structure for the following compound…
4-methyl-2-pentene
4. Name the following structures:

5. Draw (Z)-5-Chloro-3-ethly-4-hexen-2-ol
Answers
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- S. Devarajan (UCD)
- Prof. Steven Farmer (Sonoma State University)


