Objectives
After completing this section, you should be able to
- write an equation for the catalytic hydrogenation of an alkene.
- identify the product obtained from the hydrogenation of a given alkene.
- identify the alkene, the reagents, or both, required to prepare a given alkane by catalytic hydrogenation.
- describe the mechanism of the catalytic hydrogenation of alkenes.
- explain the difference between a heterogeneous reaction and a homogeneous reaction.
- recognize that other types of compounds containing multiple bonds, such as ketones, esters, nitriles and aromatic compounds, do not react with hydrogen under the conditions used to hydrogenate alkenes.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- Adams’ catalyst
- hydrogenation
Study Notes
Chemical reactions that are heterogeneous have reactants that are in at least two different phases (e.g. gas with a solid), whereas homogeneous reactions occur in a single phase (e.g. gas with another gas).
Some confusion may arise from the description of the catalyst used in the reaction between alkenes and hydrogen. Three metals—nickel, platinum and palladium—are commonly used, but a chemist cannot simply place a piece of one of these metals in a mixture of the alkene and hydrogen and get a reaction. Each metal catalyst must be prepared in a special way:
- nickel is usually used in a finely divided form called “Raney nickel.” It is prepared by reacting a Ni-Al alloy with NaOH.
- palladium is obtained commercially “supported” on an inert substance, such as charcoal, (Pd/C). The alkene is usually dissolved in ethanol when Pd/C is used as the catalyst.
- platinum is used as PtO2, Adams’ catalyst, although it is actually platinum metal that is the catalyst. The hydrogen used to add to the carbon-carbon double bond also reduces the platinum(IV) oxide to finely divided platinum metal. Ethanol or acetic acid is used as the solvent for the alkene.
Other types of compounds containing multiple bonds, such as ketones, esters, and nitriles, do not react with hydrogen under the conditions used to hydrogenate alkenes. The examples below show reduction of an alkene, but the ketone and nitrile groups present remain intact and are not reduced.

Aromatic rings are also not reduced under the conditions used to reduce alkenes, although these rings appear to contain three carbon-carbon double bonds. As you will see later, aromatic rings do not really contain any double bonds, and many chemists prefer to represent the benzene ring as a hexagon with a circle inside it

rather than as a hexagon with three alternating double bonds.
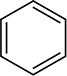
The representation of the benzene ring will be discussed further in Section 15.2.
The reaction between carbon-carbon double bonds and hydrogen provides a method of determining the number of double bonds present in a compound. For example, one mole of cyclohexene reacts with one mole of hydrogen to produce one mole of cyclohexane:

but one mole of 1,4-cyclohexadiene reacts with two moles of hydrogen to form one mole of cyclohexane:
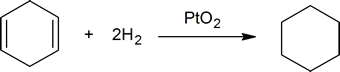
A chemist would say that cyclohexene reacts with one equivalent of hydrogen, and 1,4-cyclohexadiene reacts with two equivalents of hydrogen. If you take a known amount of an unknown, unsaturated hydrocarbon and determine how much hydrogen it will absorb, you can readily determine the number of double bonds present in the hydrocarbon (see question 2, below).
Addition of hydrogen to a carbon-carbon double bond is called hydrogenation. The overall effect of such an addition is the reductive removal of the double bond functional group. Regioselectivity is not an issue, since the same group (a hydrogen atom) is bonded to each of the double bond carbons. The simplest source of two hydrogen atoms is molecular hydrogen (H2), but mixing alkenes with hydrogen does not result in any discernible reaction. Although the overall hydrogenation reaction is exothermic, a high activation energy prevents it from taking place under normal conditions. This restriction may be circumvented by the use of a catalyst, as shown in the following diagram.
An example of an alkene addition reaction is a process called hydrogenation.In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane. Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. In other words, the energy of the product is lower than the energy of the reactant; thus it is exothermic (heat is released). The heat released is called the heat of hydrogenation, which is an indicator of a molecule’s stability.


Catalysts are substances that changes the rate (velocity) of a chemical reaction without being consumed or appearing as part of the product. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Finely divided metals, such as platinum, palladium and nickel, are among the most widely used hydrogenation catalysts. Catalytic hydrogenation takes place in at least two stages, as depicted in the diagram. First, the alkene must be adsorbed on the surface of the catalyst along with some of the hydrogen. Next, two hydrogens shift from the metal surface to the carbons of the double bond, and the resulting saturated hydrocarbon, which is more weakly adsorbed, leaves the catalyst surface. The exact nature and timing of the last events is not well understood.
As shown in the energy diagram, the hydrogenation of alkenes is exothermic, and heat is released corresponding to the ΔE (colored green) in the diagram. This heat of reaction can be used to evaluate the thermodynamic stability of alkenes having different numbers of alkyl substituents on the double bond. For example, the following table lists the heats of hydrogenation for three C5H10 alkenes which give the same alkane product (2-methylbutane). Since a large heat of reaction indicates a high energy reactant, these heats are inversely proportional to the stabilities of the alkene isomers. To a rough approximation, we see that each alkyl substituent on a double bond stabilizes this functional group by a bit more than 1 kcal/mole.
| Alkene Isomer | (CH3)2CHCH=CH2 3-methyl-1-butene |
CH2=C(CH3)CH2CH3 2-methyl-1-butene |
(CH3)2C=CHCH3 2-methyl-2-butene |
|---|---|---|---|
| Heat of Reaction ( ΔHº ) |
–30.3 kcal/mole | –28.5 kcal/mole | –26.9 kcal/mole |
From the mechanism shown here we would expect the addition of hydrogen to occur with syn-stereoselectivity. This is often true, but the hydrogenation catalysts may also cause isomerization of the double bond prior to hydrogen addition, in which case stereoselectivity may be uncertain.

Exercises
- In the reaction
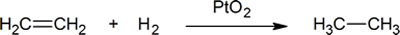
- 0.500 mol of ethene reacts with _______ mol of hydrogen. Thus a chemist might say that ethene reacts with one _______ of hydrogen.
- ethene is being _______; while _______ is being oxidized.
- the oxidation number of carbon in ethene is _______; in ethane it is _______.
- When 1.000 g of a certain triglyceride (fat) is treated with hydrogen gas in the presence of Adams’ catalyst, it is found that the volume of hydrogen gas consumed at 99.8 kPa and 25.0°C is 162 mL. A separate experiment indicates that the molar mass of the fat is 914 g mol−1. How many carbon-carbon double bonds does the compound contain?
Answers:
1.
-
-
Show Answer
-
Show Answer
-
Show Answer
-
-
Show Answer
Examples
Question
1.
Predict the products if the following alkenes were reacted with catalytic hydrogen.

Solution
1.

Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

