Learning Objectives
- Identify the general structure for an aldehyde and a ketone.
- Use common names to name aldehydes and ketones.
- Use the IUPAC system to name aldehydes and ketones.
The next functional group we consider, the carbonyl group, has a carbon-to-oxygen double bond.

Carbonyl groups define two related families of organic compounds: the aldehydes and the ketones. The carbonyl group is also found in Carboxylic acids, esters, and amides, which will be discussed in other sections.
Note
The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems.
In a ketone, two carbon groups are attached to the carbonyl carbon atom. The following general formulas, in which R represents an alkyl group and Ar stands for an aryl group, represent ketones.

In an aldehyde, at least one of the attached groups must be a hydrogen atom. The following compounds are aldehydes:

In condensed formulas, we use CHO to identify an aldehyde rather than COH, which might be confused with an alcohol. This follows the general rule that in condensed structural formulas H comes after the atom it is attached to (usually C, N, or O).

The carbon-to-oxygen double bond is not shown but understood to be present.
Because they contain the same functional group, aldehydes and ketones share many common properties, but they still differ enough to warrant their classification into two families.
Naming Aldehydes and Ketones
Both common and International Union of Pure and Applied Chemistry (IUPAC) names are frequently used for aldehydes and ketones, with common names predominating for the lower homologs. The common names of aldehydes are taken from the names of the acids into which the aldehydes can be converted by oxidation. (For more information about carboxylic acids, see Chapter 15 “Organic Acids and Bases and Some of Their Derivatives”, Section 15.2 “Carboxylic Acids: Structures and Names” through Section 15.4 “Physical Properties of Carboxylic Acids”.)

Note
The stems for the common names of the first four aldehydes are as follows:
- 1 carbon atom: form–
- 2 carbon atoms: acet–
- 3 carbon atoms: propion–
- 4 carbon atoms: butyr–
Because the carbonyl group in a ketone must be attached to two carbon groups, the simplest ketone has three carbon atoms. It is widely known as acetone, a unique name unrelated to other common names for ketones.

Generally, the common names of ketones consist of the names of the groups attached to the carbonyl group, followed by the word ketone. (Note the similarity to the naming of ethers.) Another name for acetone, then, is dimethyl ketone. The ketone with four carbon atoms is ethyl methyl ketone.

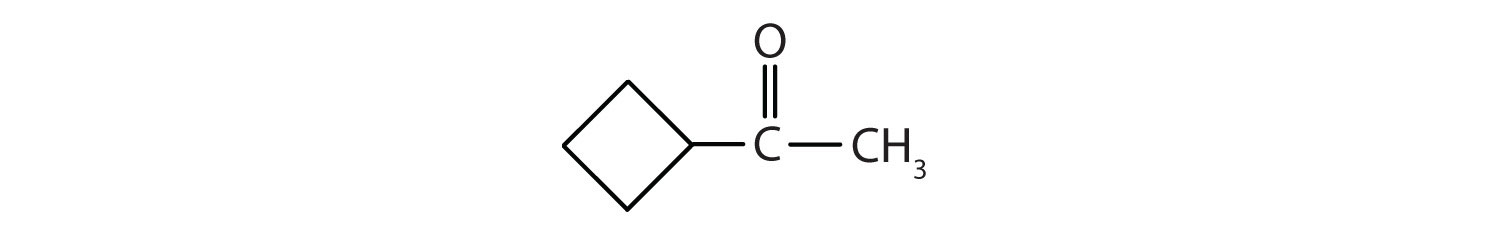
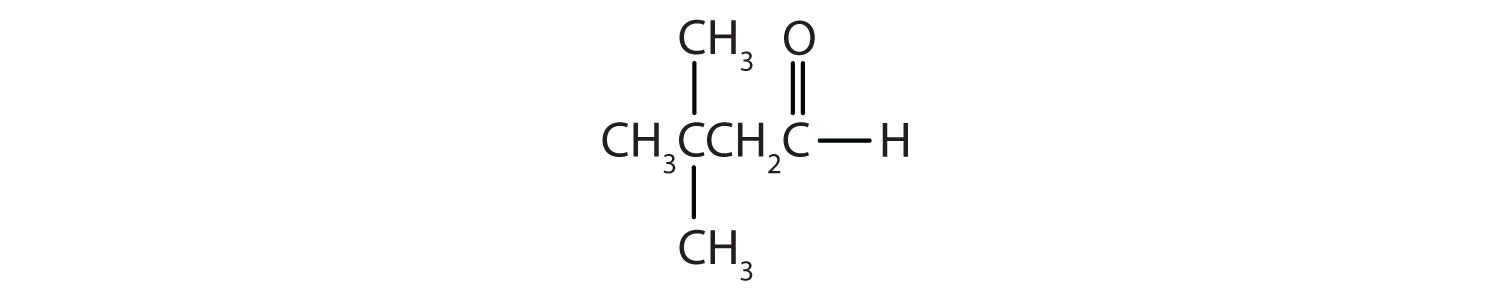
Example 6
Classify each compound as an aldehyde or a ketone. Give the common name for each ketone.
Solution
- This compound has the carbonyl group on an end carbon atom, so it is an aldehyde.
- This compound has the carbonyl group on an interior carbon atom, so it is a ketone. Both alkyl groups are propyl groups. The name is therefore dipropyl ketone.
- This compound has the carbonyl group between two alkyl groups, so it is a ketone. One alkyl group has three carbon atoms and is attached by the middle carbon atom; it is an isopropyl group. A group with one carbon atom is a methyl group. The name is therefore isopropyl methyl ketone.
Skill-Building Exercise
Classify each compound as an aldehyde or a ketone. Give the common name for each ketone.
Here are some simple IUPAC rules for naming aldehydes and ketones:
- The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (LCC) of carbon atoms that contains the functional group.
- For an aldehyde, drop the –e from the alkane name and add the ending –al. Methanal is the IUPAC name for formaldehyde, and ethanal is the name for acetaldehyde.
- For a ketone, drop the –e from the alkane name and add the ending –one. Propanone is the IUPAC name for acetone, and butanone is the name for ethyl methyl ketone.
- To indicate the position of a substituent on an aldehyde, the carbonyl carbon atom is always considered to be C1; it is unnecessary to designate this group by number.
- To indicate the position of a substituent on a ketone, number the chain in the manner that gives the carbonyl carbon atom the lowest possible number. In cyclic ketones, it is understood that the carbonyl carbon atom is C1.
Example 7
Give the IUPAC name for each compound.
Solution
- There are five carbon atoms in the LCC. The methyl group (CH3) is a substituent on the second carbon atom of the chain; the aldehyde carbon atom is always C1. The name is derived from pentane. Dropping the –e and adding the ending –al gives pentanal. The methyl group on the second carbon atom makes the name 2-methylpentanal.
- There are five carbon atoms in the LCC. The carbonyl carbon atom is C3, and there are methyl groups on C2 and C4. The IUPAC name is 2,4-dimethyl-3-pentanone.
- There are six carbon atoms in the ring. The compound is cyclohexanone. No number is needed to indicate the position of the carbonyl group because all six carbon atoms are equivalent.
Skill-Building Exercise
Give the IUPAC name for each compound.
Example 8
Draw the structure for each compound.
- 7-chlorooctanal
- 4-methyl–3-hexanone
Solution
-
The octan– part of the name tells us that the LCC has eight carbon atoms. There is a chlorine (Cl) atom on the seventh carbon atom; numbering from the carbonyl group and counting the carbonyl carbon atom as C1, we place the Cl atom on the seventh carbon atom.
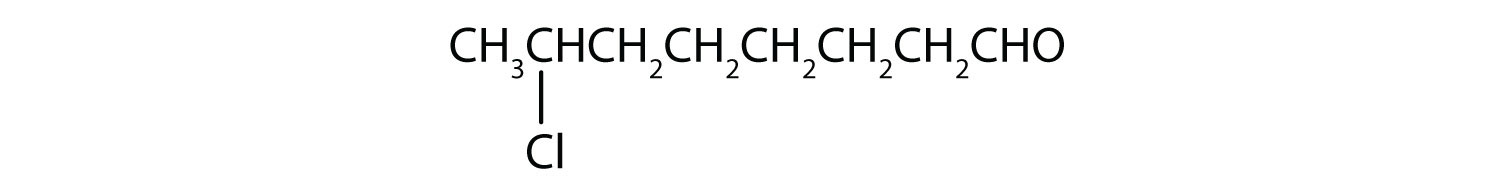
-
The hexan– part of the name tells us that the LCC has six carbon atoms. The 3 means that the carbonyl carbon atom is C3 in this chain, and the 4 tells us that there is a methyl (CH3) group at C4:

Skill-Building Exercise
Draw the structure for each compound.
-
5-bromo-3-iodoheptanal
-
5-bromo-4-ethyl-2-heptanone
Concept Review Exercises
-
Give the structure and IUPAC name for the compound that has the common name m-bromobenzaldehyde (see Figure 14.6 “Some Interesting Aldehydes” for the structure of benzaldehyde).
-
Give the IUPAC name for glyceraldehyde, (HOCH2CHOHCHO). (Hint: as a substituent, the OH group is named hydroxy.)
Key Takeaways
- The common names of aldehydes are taken from the names of the corresponding carboxylic acids: formaldehyde, acetaldehyde, and so on.
- The common names of ketones, like those of ethers, consist of the names of the groups attached to the carbonyl group, followed by the word ketone.
- Stem names of aldehydes and ketones are derived from those of the parent alkanes, using an –al ending for an aldehydes and an –one ending for a ketone.
Exercises
-
Name each compound.
-
-
Name each compound.
-

- CH3CH2CH2CH2CH2CHO
-

-

-
-
Draw the structure for each compound.
- butanal
- 2-hexanone
- p-nitrobenzaldehyde
-
Draw the structure for each compound.
- 5-ethyloctanal
- 2-chloropropanal
- 2-hydroxy-3-pentanone