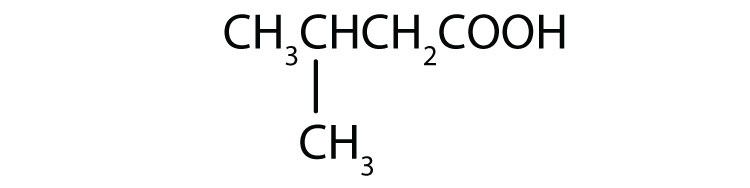The simplest carboxylic acid, formic acid (HCOOH), was first obtained by the distillation of ants (Latin formica, meaning “ant”). The bites of some ants inject formic acid, and the stings of wasps and bees contain formic acid (as well as other poisonous materials).

The next higher homolog is acetic acid, which is made by fermenting cider and honey in the presence of oxygen. This fermentation produces vinegar, a solution containing 4%–10% acetic acid, plus a number of other compounds that add to its flavor. Acetic acid is probably the most familiar weak acid used in educational and industrial chemistry laboratories.

Note
Pure acetic acid solidifies at 16.6°C, only slightly below normal room temperature. In the poorly heated laboratories of the late 19th and early 20th centuries in northern North America and Europe, acetic acid often “froze” on the storage shelf. For that reason, pure acetic acid (sometimes called concentrated acetic acid) came to be known as glacial acetic acid, a name that survives to this day.
The third homolog, propionic acid (CH3CH2COOH), is seldom encountered in everyday life. The fourth homolog, butyric acid (CH3CH2CH2COOH), is one of the most foul-smelling substances imaginable. It is found in rancid butter and is one of the ingredients of body odor. By recognizing extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives. Models of the first four carboxylic acids are shown in Figure 15.2 “Ball-and-Stick Models of Carboxylic Acids”.

Figure 15.2 Ball-and-Stick Models of Carboxylic Acids. Carboxylic acids feature a carbon atom doubly bonded to an oxygen atom and also joined to an OH group. The four acids illustrated here are formic acid (a), acetic acid (b), propionic acid (c), and butyric acid (d). Note the color scheme: blue for C, red for O, and green for H.
The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid (C6H5COOH).

The common names of carboxylic acids use Greek letters (α, β, γ, δ, and so forth), not numbers, to designate the position of substituent groups in acids. These letters refer to the position of the carbon atom in relation to the carboxyl carbon atom.

In the nomenclature system of the International Union of Pure and Applied Chemistry (IUPAC), the parent hydrocarbon is the one that corresponds to the longest continuous chain (LCC) containing the carboxyl group. The –e ending of the parent alkane is replaced by the suffix –oic and the word acid. For example, the carboxylic acid derived from pentane is pentanoic acid (CH3CH2CH2CH2COOH). As with aldehydes, the carboxyl carbon atom is counted first; numbers are used to indicate any substituted carbon atoms in the parent chain.
Note
Greek letters are used with common names; numbers are used with IUPAC names.
Example 1
Give the common and IUPAC names for each compound.
- ClCH2CH2CH2COOH
-

Skill-Building Exercise
Give the common and IUPAC names for each compound.
-
ClCH2CH2CH2CH2COOH
-
(CH3)2CHCH2CHBrCOOH
Example 2
Write the condensed structural formula for β-chloropropionic acid.
Skill-Building Exercise
-
Write the condensed structural formula for 4-bromo-5-methylhexanoic acid.
Concept Review Exercises
-
What is the IUPAC name for the straight-chain carboxylic acid with six carbon atoms?
-
The straight-chain aldehyde with five carbon atoms has the common name valeraldehyde. What is the common name of the corresponding straight-chain carboxylic acid?
Key Takeaways
- Simple carboxylic acids are best known by common names based on Latin and Greek words that describe their source (e.g., formic acid, Latin formica, meaning “ant”).
- Greek letters, not numbers, designate the position of substituted acids in the common naming convention.
- IUPAC names are derived from the LCC of the parent hydrocarbon with the –e ending of the parent alkane replaced by the suffix –oic and the word acid.
Exercises
-
Draw the structure for each compound.
- heptanoic acid
- 3-methylbutanoic acid
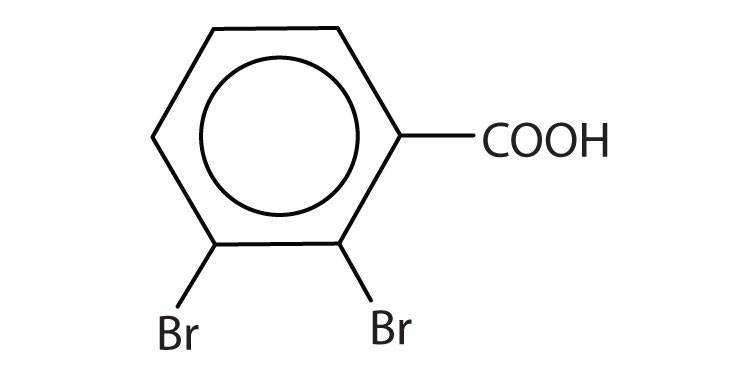
- 2,3-dibromobenzoic acid
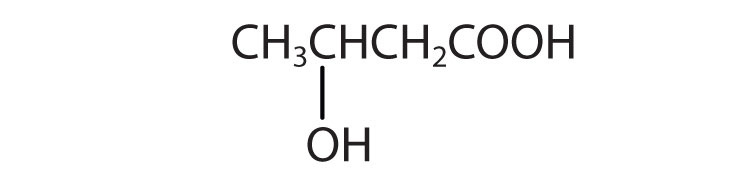
- β-hydroxybutyric acid
-
Draw the structure for each compound.
- o-nitrobenzoic acid
- p-chlorobenzoic acid
- 3-chloropentanoic acid
- α-chloropropionic acid
-
Name each compound with either the IUPAC name, the common name, or both. It may help to draw the structure first.
- (CH3)2CHCH2COOH
- (CH3)3CCH(CH3)CH2COOH
- CH2OHCH2CH2COOH
-
Name each compound with its IUPAC name.
- CH3(CH2)8COOH
- (CH3)2CHCCl2CH2CH2COOH
- CH3CHOHCH(CH2CH3)CHICOOH
Candela Citations
- The Basics of General, Organic, and Biological Chemistry v. 1.0. Provided by: Saylor Academy. Located at: https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/. License: CC BY-NC: Attribution-NonCommercial. License Terms: This text was adapted by Saylor Academy under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License without attribution as requested by the work's original creator or licensor.