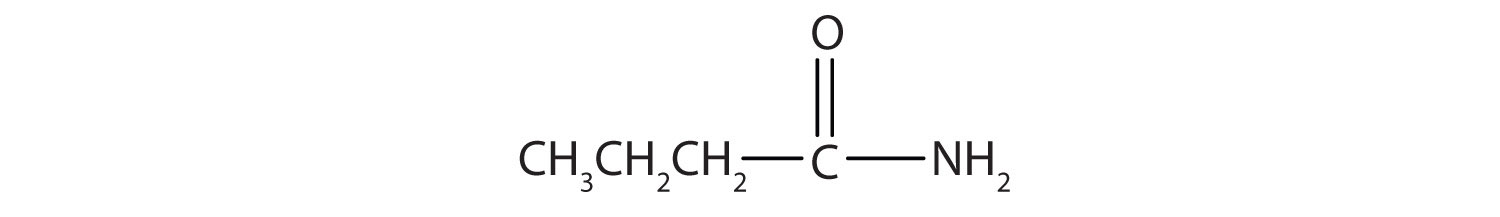Learning Objectives
- Identify the general structure for an amide.
- Identify the functional group for an amide.
- Names amides with common names.
- Name amides according to the IUPAC system.
The amide functional group has an nitrogen atom attached to a carbonyl carbon atom. If the two remaining bonds on the nitrogen atom are attached to hydrogen atoms, the compound is a simple amide. If one or both of the two remaining bonds on the atom are attached to alkyl or aryl groups, the compound is a substituted amide.

Note
The carbonyl carbon-to-nitrogen bond is called an amide linkage. This bond is quite stable and is found in the repeating units of protein molecules, where it is called a peptide linkage. (For more about peptide linkages, see Chapter 18 “Amino Acids, Proteins, and Enzymes”, Section 18.3 “Peptides”.)
Simple amides are named as derivatives of carboxylic acids. The –ic ending of the common name or the –oic ending of the International Union of Pure and Applied Chemistry (IUPAC) name of the carboxylic acid is replaced with the suffix –amide.

Example 16
Name each compound with the common name, the IUPAC name, or both.
Solution
- This amide has two carbon atoms and is thus derived from acetic acid. The OH of acetic acid is replaced by an NH2 group. The –ic from acetic (or –oic from ethanoic) is dropped, and –amide is added to give acetamide (or ethanamide in the IUPAC system).
- This amide is derived from benzoic acid. The –oic is dropped, and –amide is added to give benzamide.
Skill-Building Exercise
Name each compound with the common name, the IUPAC name, or both.
Concept Review Exercises
-
Name this compound with the common name and the IUPAC name.

-
Draw a the structural formulae for pentanamide.
Key Takeaways
- Amides have a general structure in which a nitrogen atom is bonded to a carbonyl carbon atom.
-
The functional group for an amide is as follows:

- In names for amides, the –ic acid of the common name or the –oic ending of the IUPAC for the corresponding carboxylic acid is replaced by –amide.
Exercises
-
Draw the structure for each compound.
- formamide
- hexanamide
-
Draw the structure for each compound.
- propionamide
- butanamide
-
Name each compound with the common name, the IUPAC name, or both.
-
-
Name the compound.

Candela Citations
- The Basics of General, Organic, and Biological Chemistry v. 1.0. Provided by: Saylor Academy. Located at: https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/. License: CC BY-NC: Attribution-NonCommercial. License Terms: This text was adapted by Saylor Academy under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License without attribution as requested by the work's original creator or licensor.