Learning objectives
- Describe how electrons are grouped within atoms.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement?
The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:
- Electrons in atoms can have only certain specific energies. We say that the energies of the electrons are quantized.
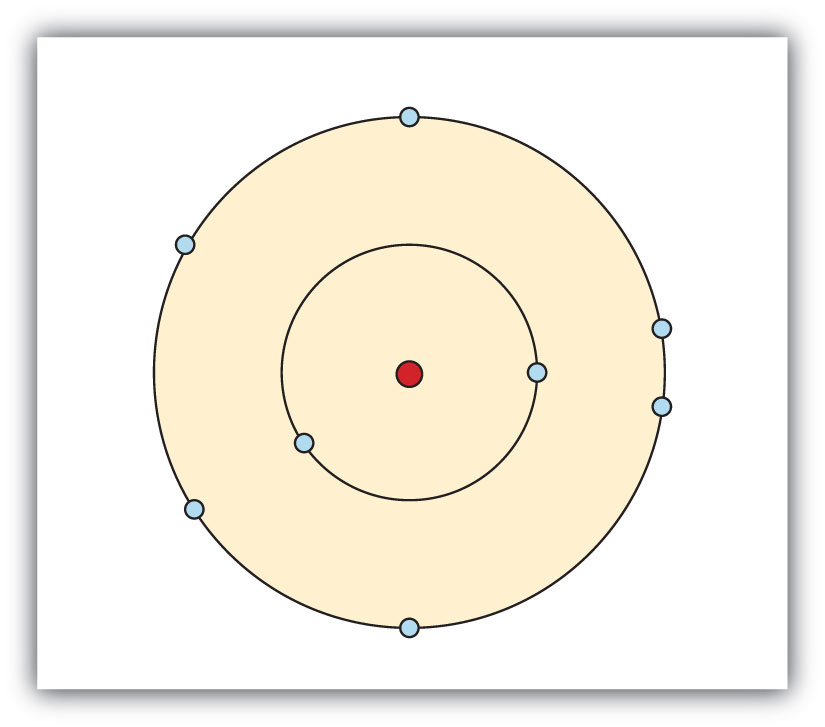
- Electrons are organized according to their energies into sets called shells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell.
- Shells are further divided into subsets of electrons called subshells. The first shell has only one subshell, the second shell has two subshells, the third shell has three subshells, and so on. The subshells of each shell are labeled, in order, with the letters s, p, d, and f. Thus, the first shell has only an s subshell, the second shell has an s and a p subshell, the third shell has s, p, and d subshells, and so forth.
- Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14.
It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1s1 to describe the electronic structure of hydrogen. This structure is called an electron configuration. Electron configurations are shorthand descriptions of the arrangements of electrons in atoms. The electron configuration of a hydrogen atom is spoken out loud as “one-ess-one.”
Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s2 (spoken as “one-ess-two”).
The 1s subshell cannot hold 3 electrons (because an s subshell can hold a maximum of 2 electrons), so the electron configuration for a lithium atom cannot be 1s3. Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell. The second shell has two subshells, s and p, which fill with electrons in that order. The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons. Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s22s1.
The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1s22s2. Now that the 2s subshell is filled, electrons in larger atoms start filling the 2p subshell. Thus, the electron configurations for the next six atoms are as follows:
B: 1s22s22p1
C: 1s22s22p2
N: 1s22s22p3
O: 1s22s22p4
F: 1s22s22p5
Ne: 1s22s22p6
With neon, the 2p subshell is completely filled. Because the second shell has only two subshells, atoms with more electrons now must begin the third shell. The third shell has three subshells, labeled s, p, and d. The d subshell can hold a maximum of 10 electrons. The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s22s22p63s23p1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms.
A fourth subshell, the f subshell, is needed to complete the electron configurations for all elements. An f subshell can hold up to 14 electrons.
Example 7
What is the electron configuration of a neutral phosphorus atom?
Skill-Building Exercise
-
What is the electron configuration of a neutral chlorine atom?
Chemistry results from interactions between the outermost shells of electrons on different atoms. Thus, it is convenient to separate electrons into two groups. Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1s22s22p2—that it has 4 valence electrons (2s22p2) and 2 core electrons (1s2).
Example 8
From the electron configuration of neutral phosphorus atoms in Example 7, how many valence electrons and how many core electrons does a neutral phosphorus atom have?
Skill-Building Exercise
-
From the electron configuration of neutral chlorine atoms (see the Skill-Building Exercise following Example 7), how many valence electrons and how many core electrons does a neutral chlorine atom have?
Concept Review Exercises
-
How are electrons organized in atoms?
-
What information does an electron configuration convey?
-
What is the difference between core electrons and valence electrons?
Key Takeaway
- Electrons are organized into shells and subshells about the nucleus of an atom.
Exercises
-
What is the maximum number of electrons that can fit in an s subshell? Does it matter what shell the s subshell is in?
-
What is the maximum number of electrons that can fit in a p subshell? Does it matter what shell the p subshell is in?
-
What is the maximum number of electrons that can fit in a d subshell? Does it matter what shell the d subshell is in?
-
What is the maximum number of electrons that can fit in an f subshell? Does it matter what shell the f subshell is in?
-
What is the electron configuration of a carbon atom?
-
What is the electron configuration of a sulfur atom?
-
What is the valence shell electron configuration of a calcium atom?
-
What is the valence shell electron configuration of a selenium atom?
-
What atom has the electron configuration 1s22s22p5?
-
What atom has the electron configuration 1s22s22p63s23p3?
-
Draw a representation of the electronic structure of an oxygen atom.
-
Draw a representation of the electronic structure of a phosphorus atom.
-
A potassium atom has ____ core electrons and ____ valence electrons.
-
A silicon atom has ____ core electrons and ____ valence electrons.