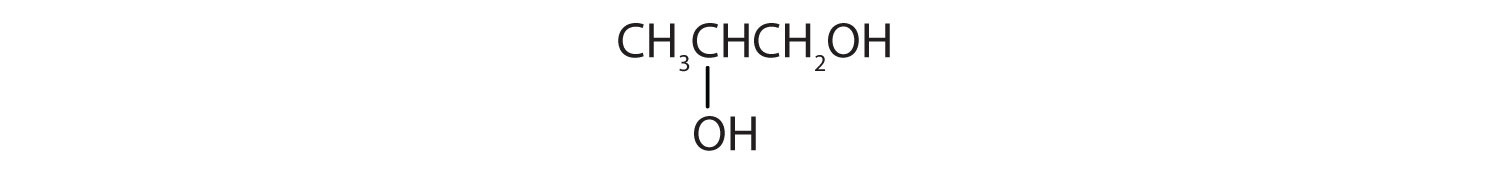Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the summary and ask yourself how they relate to the topics in the chapter.
A functional group is any atom or atom group that confers characteristic properties to a family of compounds.
The hydroxyl group (OH) is the functional group of the alcohols. The alcohols are represented by the general formula ROH. Alcohols are derived from alkanes by replacing one or more hydrogen atoms by an OH group. A primary (1°) alcohol (RCH2OH) has the OH group on a carbon atom attached to one other carbon atom; a secondary (2°) alcohol (R2CHOH) has the OH group on a carbon atom attached to two other carbon atoms; and a tertiary (3°) alcohol (R3COH) has the OH group on a carbon atom attached to three other carbon atoms.
The ability to engage in hydrogen bonding greatly increases the boiling points of alcohols compared to hydrocarbons of comparable molar mass. Alcohols can also engage in hydrogen bonding with water molecules, and those with up to about four carbon atoms are soluble in water.
Many alcohols can be synthesized by the hydration of alkenes. Common alcohols include methanol, ethanol, and isopropyl alcohol. Methanol is quite poisonous. It can cause blindness or even death. Ethanol can be prepared from ethylene or made by fermentation. It is the “alcohol” in alcoholic beverages. On occasion, people drink methanol by mistake, thinking it is the beverage alcohol. On occasion, unscrupulous bootleggers, sell methanol to unsuspecting customers. In either case, the results are often tragic.
Rubbing alcohol is usually a 70% aqueous solution of isopropyl alcohol. Ethanol is also used in some rubbing alcohol formulations.
When water is removed from an alcohol in a dehydration step, the result is either an alkene or an ether, depending on the reaction conditions. Primary alcohols are oxidized to aldehydes or carboxylic acids, and secondary alcohols are oxidized to ketones. Tertiary alcohols are not easily oxidized.
Alcohols containing two OH groups on adjacent carbon atoms are called glycols.
Phenols (ArOH) are compounds having the OH group attached to an aromatic ring.
Ethers (ROR′, ROAr, ArOAr) are compounds in which an oxygen atom is joined to two organic groups.
The carbonyl group, a carbon-to-oxygen double bond, is the defining feature of aldehydes and ketones. In aldehydes at least one bond on the carbonyl group is a carbon-to-hydrogen bond; in ketones, both available bonds on the carbonyl carbon atom are carbon-to-carbon bonds. Aldehydes are synthesized by the oxidation of primary alcohols. The aldehyde can be further oxidized to a carboxylic acid. Ketones are prepared by the oxidation of secondary alcohols. Mild oxidizing agents oxidize aldehydes to carboxylic acids. Ketones are not oxidized by these reagents.
A thiol is a compound with an SH functional group.
Additional Exercises
-
Describe two ways that ethanol can be prepared. Which method is used to produce alcoholic beverages?
-
Give the structure of the alkene from which isopropyl alcohol is made by reaction with water in an acidic solution.
-
Ethanol is used as a solvent for some drugs that are not soluble in water. Why is methanol not used in medicines?
-
Give the structure of the alkene that is made from tert-butyl alcohol [(CH3)3COH] by reaction with water in an acidic solution.
-
Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown):
- CH3OH → HCHO
- CH3CHOHCH3 → CH3CH=CH2
- CH2=CHCH2CH3 → CH3CHOHCH2CH3
-
Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown.):
- CH3CHOHCH3 → CH3COCH3
- HOOCCH=CHCOOH → HOOCCH2CHOHCOOH
- 2 CH3OH → CH3OCH3
-
Why is methanol so much more toxic to humans than ethanol?
-
Each of the four isomeric butyl alcohols is treated with potassium dichromate (K2Cr2O7) in acid. Give the product (if any) expected from each reaction.
-
Draw the structures and give IUPAC names for the four isomeric aldehydes having the formula C5H10O.
-
Write an equation for the reaction of phenol with aqueous NaOH.
-
Write an equation for the ionization of phenol in water.
-
Draw the structures and give the common and IUPAC names for the three isomeric ketones having the formula C5H10O.
-
As we shall see in Chapter 16 “Carbohydrates”, 2,3-dihydroxypropanal and 1,3-dihydroxyacetone are important carbohydrates. Draw their structures.
-
Glutaraldehyde (pentanedial) is a germicide that is replacing formaldehyde as a sterilizing agent. It is less irritating to the eyes, the nose, and skin. Write the condensed structural formula of glutaraldehyde.
-
Why does the oxidation of isopropyl alcohol give a ketone, whereas the oxidation of isobutyl alcohol gives an aldehyde?
-
Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary (1°), secondary (2°), or tertiary (3°).
- CH3CH2CH2OH
-

-

-

-
Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary, secondary, or tertiary.
- CH3CH2OCH2CH3
-

-

-

-
Tell whether each compound forms an acidic, a basic, or a neutral solution in water.
-
-
When water is added to ethylene in the presence of an acid catalyst, only one product—ethanol—is possible. However, when water is added to propylene, two products are possible—1-propanol and 2-propanol—but only 2-propanol is formed. In 1870, the Russian chemist Vladimir V. Markovnikov proposed a rule to predict the products of such reactions: Considering water to be HOH, the hydrogen atom of water goes on the carbon atom (of the two involved in the double bond) that has the most hydrogen atoms already bonded to it. The OH group goes on the carbon atom with fewer hydrogen atoms. Use Markovnikov’s rule to predict the product of the addition of water to each compound.
- 2-methylpropene
- 1-butene
- 2-methyl-1-pentene
- 2-methyl-2-pentene
-
Ethyl alcohol, like rubbing alcohol (isopropyl alcohol), is often used for sponge baths. What property of alcohols makes them useful for this purpose?
-
In addition to ethanol, the fermentation of grain produces other organic compounds collectively called fusel oils (FO). The four principal FO components are 1-propanol, isobutyl alcohol, 3-methyl-1-butanol, and 2-methyl-1-butanol. Draw a structure for each. (FO is quite toxic and accounts in part for hangovers.)
-
Draw and name the isomeric ethers that have the formula C5H12O.
-
Menthol is an ingredient in mentholated cough drops and nasal sprays. It produces a cooling, refreshing sensation when rubbed on the skin and so is used in shaving lotions and cosmetics. Thymol, the aromatic equivalent of menthol, is the flavoring constituent of thyme.

- To what class of compounds does each belong?
- Give an alternate name for thymol.
-
Write the equation for the production of ethanol by the addition of water to ethylene. How much ethanol can be made from 14.0 kg of ethylene?
-
Methanol is not particularly toxic to rats. If methanol were newly discovered and tested for toxicity in laboratory animals, what would you conclude about its safety for human consumption?
-
The amino acid cysteine has the formula HSCH2CH(NH2)COOH. What is the sulfur-containing functional group in the cysteine molecule?
-
The amino acid methionine has the formula CH3SCH2CH2CH(NH2)COOH. What functional groups are in methionine?
-
Tetrahydrocannabinol is the principal active ingredient in marijuana. What functional groups are present in this molecule?