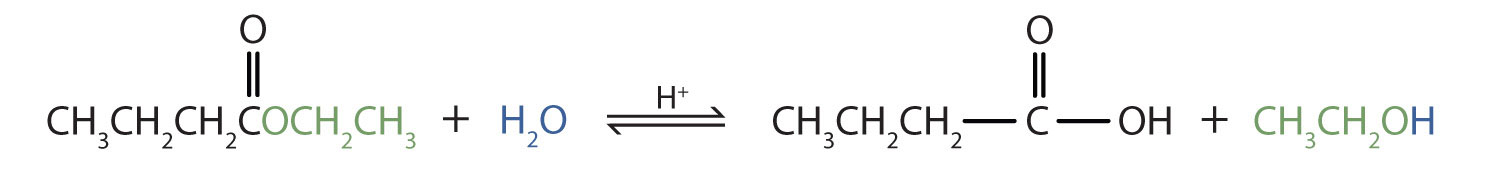Acidic hydrolysis is simply the reverse of esterification. The ester is heated with a large excess of water containing a strong-acid catalyst. Like esterification, the reaction is reversible and does not go to completion.

As a specific example, butyl acetate and water react to form acetic acid and 1-butanol. The reaction is reversible and does not go to completion.

Example 7
Write an equation for the acidic hydrolysis of ethyl butyrate (CH3CH2CH2COOCH2CH3) and name the products.
Skill-Building Exercise
-
Write an equation for the acidic hydrolysis of methyl butanoate and name the products.
When a base (such as sodium hydroxide [NaOH] or potassium hydroxide [KOH]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol. Because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponificationThe hydrolysis of fats and oils in the presence of a base to make soap. (Latin sapon, meaning “soap,” and facere, meaning “to make”). In a saponification reaction, the base is a reactant, not simply a catalyst. The reaction goes to completion:

As a specific example, ethyl acetate and NaOH react to form sodium acetate and ethanol:

Example 8
Write an equation for the hydrolysis of methyl benzoate in a potassium hydroxide solution.
Skill-Building Exercise
-
Write the equation for the hydrolysis of ethyl propanoate in a sodium hydroxide solution.
Concept Review Exercises
-
How do acidic hydrolysis and basic hydrolysis of an ester differ in terms of
- products obtained?
- the extent of reaction?
-
What is saponification?
Key Takeaways
- Hydrolysis is a most important reaction of esters.
- Acidic hydrolysis of an ester gives a carboxylic acid and an alcohol.
- Basic hydrolysis of an ester gives a carboxylate salt and an alcohol.
Exercises
-
Write an equation for the acid-catalyzed hydrolysis of ethyl acetate.
-
Write an equation for the base-catalyzed hydrolysis of ethyl acetate.
-
Complete each equation.
-
-
Complete each equation.
- (CH3)2CHCOOCH2CH3 + H2O ⇄H+
- CH3COOCH(CH3)2 + KOH(aq) →
Candela Citations
- The Basics of General, Organic, and Biological Chemistry v. 1.0. Provided by: Saylor Academy. Located at: https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/. License: CC BY-NC: Attribution-NonCommercial. License Terms: This text was adapted by Saylor Academy under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License without attribution as requested by the work's original creator or licensor.