Physical organic chemistry is mainly centered on two main components: Kinetics and thermodynamics. These should normally have been covered in your general chemistry class, but here we will apply these quantitative tools to help us understand what is happening in an organic reaction.
In this context, thermodynamics deals with energy and entropy changes that take place during a chemical reaction. It also deals with equilibrium constants for reversible reactions.
In contrast, kinetics deals with rates of reactions and energy barriers/activation energies, It is less useful for reversible reactions, but can be very useful for understanding irreversible reactions.
| Field | Questions | Usual parameters |
| Thermodynamics | Will a reaction occur? If so, how far will the reaction go? | ΔG, ΔH, ΔS, Keq |
| Kinetics | How quickly wiil the reaction go? | [X] (concn. of X), k (rate constant), Ea, ΔG‡. |
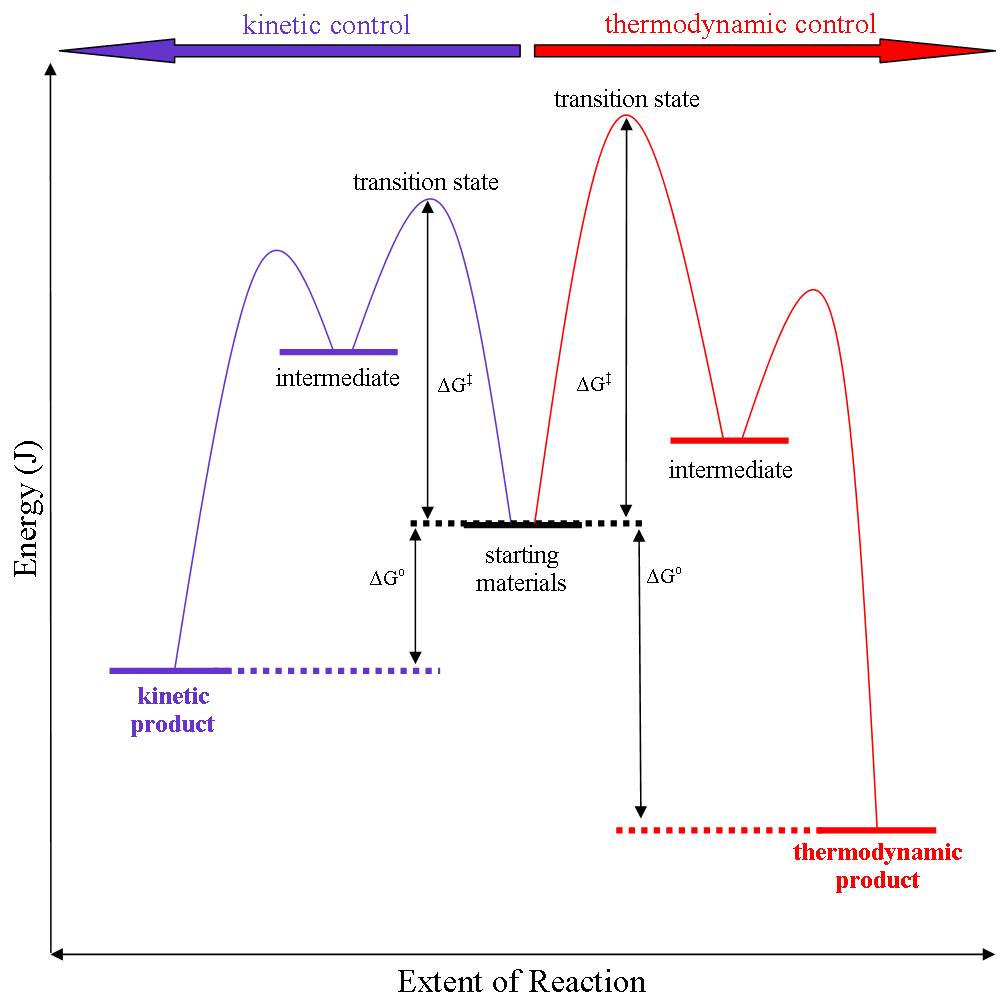
In some cases, the course of a reaction may be determined by the stability of the favored product. This is referred to as thermodynamic control, and it implies that the reaction can happen easily as most reactant molecules have enough energy to cross the energy barrier (free energy of activation, ΔG‡). In other cases we have kinetic control, where the course of a reaction may be determined by which product has the lowest energy barrier, which implies that the molecules barely have enough energy to cross even the lowest barrier. Both of these scenarios can be seen in the diagram below:
Candela Citations
- Authored by: Martin Walker. Provided by: SUNY Potsdam. Located at: http://directory.potsdam.edu/?function=user=walkerma. License: CC BY-SA: Attribution-ShareAlike
- Generalised energy profile diagram for kinetic versus thermodynamic product reaction. Authored by: User:Nick024. Located at: https://commons.wikimedia.org/wiki/File:Thermodyamic_versus_kinetic_control.png. Project: Wikimedia Commons. License: CC0: No Rights Reserved