Representing electron flow – “arrow pushing”
In organic chemistry, it is important to understand the concept of electron flow. In polar reaction mechanisms, such as the nucleophilic substitution reactions of haloalkanes, electron flow will be designated by arrows indicating the movement of electrons from electron rich regions to electron poor regions.
Introduction
In considering this concept, we must look at the two types of arrows provided in the mechanisms shown below. The curved arrows indicate the movement of electrons. The first type of arrow, shown in pink, originates from the electron pair of the nucleophile and extends to the electrophilic carbon of the haloalkane. This type of movement does not indicate that electrons leave the nucleophile; rather, it means that electrons become shared between the nucleophile and the electrophilic atom.
The second type of curved arrow, also shown in pink, originates from the R-X bond and extends to the halogen. This indicates cleavage of the bond, whereby the electron pair becomes separated from R, the electrophilic carbon, and ends up on the halogen atom.
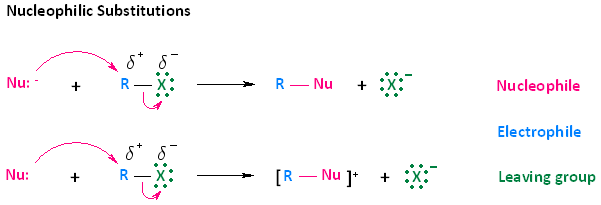
While we are using the concept of nucleophilic substitution mechanisms to explain electron flow, it is very important to understand that this concept will be applied in nearly all the mechanisms you learn throughout your course of study. The simplest way to think about this in any mechanism you learn is that electrons will be pushed from an electron rich species or site to an electron poor species or site, and the direction of the curved arrow will indicate this.

Rules for standard electron flow (electron pairs)
Although these curved arrows can seem overly complex, in fact the meaning is simple – the movement of a pair of electrons. There are some common patterns, but these “rules” should be understood rather than memorized. They are a useful check when answering a mechanism question on an exam or homework.
Rule 1: Curved arrows show movement of electron pairs, not atoms
Rule 2: Electron flow is from electron–rich (nucleophile) to electron–poor (electrophile) 
Rule 4: The overall charge stays the same, i.e. it’s the same for all the products at the end as it is for all reactants at the start.
Rule 5: The atoms for the products should match with the reactants.
Examples
