Classify different types of atomic bonds
When atoms bond together, they create elements. The different types of bonds (ionic and covalent, polar and non-polar) have an impact on the elements they create. Understanding the types of bonds that create things can help us understand those things themselves.
Learning Objectives
- Describe the characteristics of ionic bonds and identify common ions
- Describe the characteristics of covalent bonds and differentiate between polar and non-polar bonds
- Model a Hydrogen bond and identify its unique qualities
Ionic Bonds
Some atoms are more stable when they gain or lose an electron (or possibly two) and form ions. This fills their outermost electron shell and makes them energetically more stable. Because the number of electrons does not equal the number of protons, each ion has a net charge. Cations are positive ions that are formed by losing electrons. Negative ions are formed by gaining electrons and are called anions. Anions are designated by their elemental name being altered to end in “-ide”: the anion of chlorine is called chloride, and the anion of sulfur is called sulfide, for example.
This movement of electrons from one element to another is referred to as electron transfer. As Figure 1 illustrates, sodium (Na) only has one electron in its outer electron shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. It is now referred to as a sodium ion. Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. It is now referred to as a chloride ion. In this example, sodium will donate its one electron to empty its shell, and chlorine will accept that electron to fill its shell. Both ions now satisfy the octet rule and have complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium cation) or –1 (chloride anion) charge. Note that these transactions can normally only take place simultaneously: in order for a sodium atom to lose an electron, it must be in the presence of a suitable recipient like a chlorine atom.
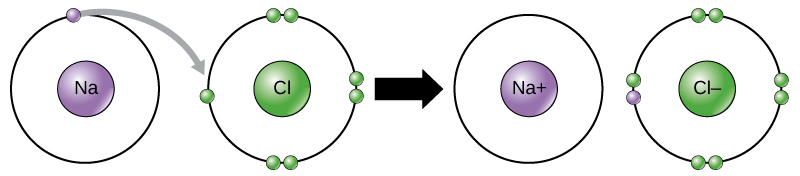
Figure 1. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. Ionic bonds are formed between ions with opposite charges. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge.
Certain salts are referred to in physiology as electrolytes (including sodium, potassium, and calcium), ions necessary for nerve impulse conduction, muscle contractions and water balance. Many sports drinks and dietary supplements provide these ions to replace those lost from the body via sweating during exercise.
Video Review
This video shows how ionic compounds form from anions and cations.
Covalent Bonds
Another way the octet rule can be satisfied is by the sharing of electrons between atoms to form covalent bonds. These bonds are stronger and much more common than ionic bonds in the molecules of living organisms. Covalent bonds are commonly found in carbon-based organic molecules, such as our DNA and proteins. Covalent bonds are also found in inorganic molecules like H2O, CO2, and O2. One, two, or three pairs of electrons may be shared, making single, double, and triple bonds, respectively. The more covalent bonds between two atoms, the stronger their connection. Thus, triple bonds are the strongest.
The strength of different levels of covalent bonding is one of the main reasons living organisms have a difficult time in acquiring nitrogen for use in constructing their molecules, even though molecular nitrogen, N2, is the most abundant gas in the atmosphere. Molecular nitrogen consists of two nitrogen atoms triple bonded to each other and, as with all molecules, the sharing of these three pairs of electrons between the two nitrogen atoms allows for the filling of their outer electron shells, making the molecule more stable than the individual nitrogen atoms. This strong triple bond makes it difficult for living systems to break apart this nitrogen in order to use it as constituents of proteins and DNA.
The formation of water molecules provides an example of covalent bonding. The hydrogen and oxygen atoms that combine to form water molecules are bound together by covalent bonds. The electron from the hydrogen splits its time between the incomplete outer shell of the hydrogen atoms and the incomplete outer shell of the oxygen atoms. To completely fill the outer shell of oxygen, which has six electrons in its outer shell but which would be more stable with eight, two electrons (one from each hydrogen atom) are needed: hence the well-known formula H2O. The electrons are shared between the two elements to fill the outer shell of each, making both elements more stable.
View this short video to see an animation of ionic and covalent bonding.
Polar Covalent Bonds
There are two types of covalent bonds: polar and nonpolar. In a polar covalent bond, shown in Figure 2, the electrons are unequally shared by the atoms and are attracted more to one nucleus than the other. Because of the unequal distribution of electrons between the atoms of different elements, a slightly positive (δ+) or slightly negative (δ–) charge develops. This partial charge is an important property of water and accounts for many of its characteristics.
Water is a polar molecule, with the hydrogen atoms acquiring a partial positive charge and the oxygen a partial negative charge. This occurs because the nucleus of the oxygen atom is more attractive to the electrons of the hydrogen atoms than the hydrogen nucleus is to the oxygen’s electrons. Thus oxygen has a higher electronegativity than hydrogen and the shared electrons spend more time in the vicinity of the oxygen nucleus than they do near the nucleus of the hydrogen atoms, giving the atoms of oxygen and hydrogen slightly negative and positive charges, respectively. Another way of stating this is that the probability of finding a shared electron near an oxygen nucleus is more likely than finding it near a hydrogen nucleus. Either way, the atom’s relative electronegativity contributes to the development of partial charges whenever one element is significantly more electronegative than the other, and the charges generated by these polar bonds may then be used for the formation of hydrogen bonds based on the attraction of opposite partial charges. (Hydrogen bonds, which are discussed in detail below, are weak bonds between slightly positively charged hydrogen atoms to slightly negatively charged atoms in other molecules.) Since macromolecules often have atoms within them that differ in electronegativity, polar bonds are often present in organic molecules.
Nonpolar Covalent Bonds

Figure 2. Whether a molecule is polar or nonpolar depends both on bond type and molecular shape. Both water and carbon dioxide have polar covalent bonds, but carbon dioxide is linear, so the partial charges on the molecule cancel each other out.
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O2) is nonpolar because the electrons will be equally distributed between the two oxygen atoms.
Another example of a nonpolar covalent bond is methane (CH4), also shown in Figure 2. Carbon has four electrons in its outermost shell and needs four more to fill it. It gets these four from four hydrogen atoms, each atom providing one, making a stable outer shell of eight electrons. Carbon and hydrogen do not have the same electronegativity but are similar; thus, nonpolar bonds form. The hydrogen atoms each need one electron for their outermost shell, which is filled when it contains two electrons. These elements share the electrons equally among the carbons and the hydrogen atoms, creating a nonpolar covalent molecule.
Watch this video for another explanation of covalent bonds and how they form:
Hydrogen Bonds
Ionic and covalent bonds between elements require energy to break. Iconic bonds are not as strong as covalent, which determines their behavior in biological systems. However, not all bonds are ionic or covalent bonds. Weaker bonds can also form between molecules. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist. Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and DNA, the building block of cells.
When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen’s electron is pulled more strongly toward the other element and away from the hydrogen. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When this happens, a weak interaction occurs between the δ+ of the hydrogen from one molecule and the δ– charge on the more electronegative atoms of another molecule, usually oxygen or nitrogen, or within the same molecule. This interaction is called a hydrogen bond. This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. Hydrogen bonds are also responsible for zipping together the DNA double helix.
Check Your Understanding
Answer the question(s) below to see how well you understand the topics covered in the previous section. This short quiz does not count toward your grade in the class, and you can retake it an unlimited number of times.
Use this quiz to check your understanding and decide whether to (1) study the previous section further or (2) move on to the next section.
Candela Citations
- Introduction to Atomic Bonds. Authored by: Shelli Carter and Lumen Learning. Provided by: Lumen Learning. License: CC BY: Attribution
- Ionic Bonding. Authored by: Bozeman Science. Located at: https://youtu.be/hiyTfhjeF_U. License: All Rights Reserved. License Terms: Standard YouTube License
- Ionic and covalent bonding animation. Authored by: Edu2000.org. Located at: https://youtu.be/QqjcCvzWwww. License: All Rights Reserved. License Terms: Standard YouTube License
- Covalent Bonding. Authored by: Bozeman Science. Located at: https://youtu.be/Mo4Vfqt5v2A. License: All Rights Reserved. License Terms: Standard YouTube License
- Water: A Polar Molecule. Authored by: Bozeman Science. Located at: https://youtu.be/iOOvX0jmhJ4. License: All Rights Reserved. License Terms: Standard YouTube License