Democritus’ Idea of the Atom
Learning Objectives
- Describe how the Greek philosophers approached nature.
- Describe the discussion about matter.
- Describe the contribution Democritus made to our understanding of matter.
What would the philosophers do?
People enjoy getting together to discuss things, whether it is how your favorite sports team is doing, what the best new movie is, the current politics, or any number of other topics. Often the question is raised about who is right and who is wrong. If the football game is to be played this coming weekend, all we can do is offer opinions as to its outcome. The game has not been played yet, so we don’t know who will actually win.

The ancient Greek philosophers did a lot of discussing, with part of their conversations concerning the physical world and its composition. There were different opinions about what made up matter. Some felt one thing was true while others believed another set of ideas. Since these scholars did not have laboratories and had not developed the idea of the experiment, they were left to debate. Whoever could offer the best argument was considered right. However, often the best argument had little to do with reality.

Figure 1. Into how small of pieces can you divide a grain of sand?
One of the on-going debates had to do with sand. The question posed was: into how small of pieces can you divide a grain of sand? The prevailing thought at the time, pushed by Aristotle, was that the grain of sand could be divided indefinitely, that you could always get a smaller particle by dividing a larger one and there was no limit to how small the resulting particle could be.
Since Aristotle was such an influential philosopher, very few people disagreed with him. However, there were some philosophers who believed that there was a limit to how small a grain of sand could be divided. One of these philosophers was Democritus (~460-~370 B.C.), often referred to as the “laughing philosopher” because of his emphasis on cheerfulness. He taught that there were substances called atoms and that these atoms made up all material things. The atoms were unchangeable, indestructible, and always existed.

Figure 2. Democritus.
The word “ atom ” comes from the Greek atomos and means “indivisible.” The atomists of the time (Democritus being one of the leading atomists) believed there were two realities that made up the physical world: atoms and void. There was an infinite number of atoms, but different types of atoms had different sizes and shapes. The void was the empty space in which the atoms moved and collided with one another. When these atoms collided with one another, they might repel each other or they might connect in clusters, held together by tiny hooks and barbs on the surface of the atoms.
Aristotle disagreed with Democritus and offered his own idea of the composition of matter. According to Aristotle, everything was composed of four elements: earth, air, fire, and water. The theory of Democritus explained things better, but Aristotle was more influential, so his ideas prevailed. We had to wait almost two thousand years before scientists came around to seeing the atom as Democritus did.
How right was Democritus?
It is very interesting that Democritus had the basic idea of atoms, even though he had no experimental evidence to support his thinking. We now know more about how atoms hold together in “clusters” (compounds), but the basic concept existed over two thousand years ago. We also know that atoms can be further subdivided, but there is still a lower limit to how small we can break up that grain of sand.
Summary
- Greek philosophers debated about many things.
- Aristotle and others believed that a grain of sand could be divided indefinitely.
- Democritus believed there was a lower limit to the dividing of a grain of sand.
Practice
Use the link below to answer the following questions:
http://plato.stanford.edu/entries/democritus/
- Who influenced the thinking of Democritus?
- Who were the atomists?
- How did Democritus explain how we saw objects?
- What type of atom did Democritus believe the soul was composed of?
Review
- How did the ancient Greek philosophers spend their time?
- What approach did they not have for studying nature?
- Who was the most influential philosopher of that time?
- What was the major contribution Democritus made to the thinking of his day?
- List characteristics of atoms according to Democritus.
Glossary
philosopher: People who do a lot of discussing and debate, with part of their conversations concerning the physical world and its composition.
atom: The philosopher Democritus (~460-~370 B.C.), taught that there were substances called atoms and that these atoms made up all material things. The atoms were unchangeable, indestructible, and always existed.
Conservation of Mass
Learning Objectives
State the law of conservation of mass.
Have you ever lost a screw?
 The following situation happens all too often. You have taken apart a piece of equipment to clean it up. When you put the equipment back together, somehow you have an extra screw or two. Or you find out that a screw is missing that was a part of the original equipment. In either case, you know something is wrong. You expect to end up with the same amount of material that you started with, not with more or less than what you had originally.
The following situation happens all too often. You have taken apart a piece of equipment to clean it up. When you put the equipment back together, somehow you have an extra screw or two. Or you find out that a screw is missing that was a part of the original equipment. In either case, you know something is wrong. You expect to end up with the same amount of material that you started with, not with more or less than what you had originally.
Law of Conservation of Mass
By the late 1700s, chemists accepted the definition of an element as a substance that cannot be broken down into a simpler substance by ordinary chemical means. It was also clear that elements combine with one another to form more complex substances called compounds. The chemical and physical properties of these compounds are different than the properties of the elements from which they were formed. There were questions about the details of these processes.
In the 1790s, a greater emphasis began to be placed on the quantitative analysis of chemical reactions. Accurate and reproducible measurements of the masses of reacting elements and the compounds they form led to the formulation of several basic laws . One of these is called the law of conservation of mass , which states that during a chemical reaction, the total mass of the products must be equal to the total mass of the reactants . In other words, mass cannot be created or destroyed during a chemical reaction, but is always conserved.
As an example, consider the reaction between silver nitrate and sodium chloride. These two compounds will dissolve in water to form silver chloride and sodium nitrate. The silver chloride does not dissolve in water, so it forms a solid that we can filter off. When we evaporate the water, we can recover the sodium nitrate formed. If we react 58.5 grams of sodium chloride with 169.9 grams of silver nitrate, we start with 228.4 grams of materials. After the reaction is complete and the materials separated, we find that we have formed 143.4 grams of silver chloride and 85.0 grams of sodium nitrate, giving us a total mass of 228.4 grams for the products. So, the total mass of reactants equals the total mass of products, a proof of the law of conservation of mass.
Watch this video for a demonstration on the Law of Conservation of Mass
Summary
- The law of conservation of mass states that, during a chemical reaction, the total mass of the products must be equal to the total mass of the reactants.
Practice
Use the link below to answer the following questions:
https://legacy.etap.org/demo/grade8_science/lesson5/instruction1tutor.html
- If you want to say something about chemical reactions, what would you use?
- What does the Law of Conservation of Mass mean?
- How much oxygen gas would I need if I react six molecules of hydrogen?
- How many molecules of water would be formed?
Review
- State the Law of Conservation of Mass.
- What does this law mean?
Glossary
- law: Accurate and reproducible measurements of the masses of reacting elements and the compounds they form led to the formulation of several basic laws.
- conservation of mass: States that during a chemical reaction, the total mass of the products must be equal to the total mass of the reactants.
- reactant: A substance that undergoes change in a chemical reaction.
- product: The result of a chemical reaction.
Law of Definite Proportions
Learning Objectives
State the law of definite proportions.
Examples
 We use electricity for many purposes, from cooking to powering our televisions to charging our cell phones. Wherever we travel in the United States, we want electricity to be available. What we also want (although we usually don’t think about it) is for the electricity supply to be the same wherever we go. We want the same voltage (110 volts for the U.S.) to come from the outlet to whatever we plug in. If the voltage is less, the system will not work. If it is more, the equipment will be damaged. We want a definite amount of voltage – no more and no less.
We use electricity for many purposes, from cooking to powering our televisions to charging our cell phones. Wherever we travel in the United States, we want electricity to be available. What we also want (although we usually don’t think about it) is for the electricity supply to be the same wherever we go. We want the same voltage (110 volts for the U.S.) to come from the outlet to whatever we plug in. If the voltage is less, the system will not work. If it is more, the equipment will be damaged. We want a definite amount of voltage – no more and no less.

Figure 3. Water.
The discovery that mass was always conserved in chemical reactions was soon followed by the law of definite proportions , which states that a given chemical compound always contains the same elements in the exact same proportions by mass. As an example, any sample of pure water contains 11.19% hydrogen and 88.81% oxygen by mass. It does not matter where the sample of water came from or how it was prepared. Its composition, like that of every other compound, is fixed.
Another example is carbon dioxide. This gas is produced from a variety of reactions, often by the burning of materials. The structure of the gas consists of one atom of carbon and two atoms of oxygen. Carbon dioxide production is of interest in many areas, from the amount we breather out to the amount of the gas produced by burning wood or fossil fuels. By knowing the exact composition of carbon dioxide, we can make predictions as to the effects of different chemical processes.

Figure 4. Carbon dioxide is produced during the burning process.
Summary
- The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass.
Practice
Watch the video and answer the questions:
- When was the law of definite proportions developed?
- Who proposed this law?
- How many hydrogen atoms are there in a molecule of water?
- How many oxygen atoms are there in a molecule of water?
Review
- State the law of definite proportions.
- Will the composition of water vary depending on its source?
- Why is this law important?
Glossary
law of definite proportions: States that a given chemical compound always contains the same elements in the exact same proportions by mass.
Law of Multiple Proportions
Learning Objectives
State the law of multiple proportions.
What are the similarities and differences between a unicycle and a bicycle?
 Just from the words themselves, the astute Latin-speaking scholar can tell that, whatever it is made of, the unicycle has one of them ( uni = “one”) and the bicycle has two ( bi = “two”). From the picture to the right, we get additional information that helps us tell the two apart.
Just from the words themselves, the astute Latin-speaking scholar can tell that, whatever it is made of, the unicycle has one of them ( uni = “one”) and the bicycle has two ( bi = “two”). From the picture to the right, we get additional information that helps us tell the two apart.
The unicycle has one wheel and the bicycle has two. In particular, they are made up of the same materials and the only significant difference is the number of wheels on the two vehicles.
Now: how many wheels on a tricycle?
Once the idea that elements combined in definite proportions to form compounds was established, experiments also began to demonstrate that the same pairs of certain elements could combine to form more than one compound. Consider the elements carbon and oxygen. Combined in one way, they form the familiar compound called carbon dioxide. In every sample of carbon dioxide, there is 32.0 g of oxygen present for every 12.0 g of carbon. By dividing 32.0 by 12.0, this simplifies to a mass ratio of oxygen to carbon of 2.66 to 1. There is another compound that forms from the combination of carbon and oxygen called carbon monoxide. Every sample of carbon monoxide contains 16.0 g of oxygen for every 12.0 g of carbon. This is a mass ratio of oxygen to carbon of 1.33 to 1. In the carbon dioxide, there is exactly twice as much oxygen present as there is in the carbon monoxide. This example illustrates the law of multiple proportions : Whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.

Figure 5. Carbon can form two different compounds with oxygen.
In carbon monoxide, on the left, there is 1.333 g of oxygen for every 1 g of carbon. In carbon dioxide, on the right, there is 2.666 g of oxygen for every gram of carbon. So the ratio of oxygen in the two compounds is 1:2, a small whole number ratio.
The difference between carbon monoxide and carbon dioxide is significant. Carbon monoxide is a deadly gas, formed from the incomplete combustion of some carbon-containing materials (such as wood and gasoline). This compound will attach to hemoglobin in the red blood cell and block the binding of oxygen to those cells. If oxygen does not bind, it cannot be carried to the cells of the body where it is needed and death can occur. Carbon dioxide, on the other hand, is not toxic like carbon monoxide is. However, it can displace oxygen in systems since it is heavier. Carbon dioxide fire extinguishers cut off the flow of oxygen in a fire, putting out the fire.
Summary
- The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
Practice
Watch the video at the link below or read the transcript and answer the following questions:
- What is the carbon:oxygen ratio in carbon monoxide?
- What is the carbon:oxygen ratio in carbon dioxide?
- What is the hydrogen:oxygen ratio in water?
- What is the hydrogen:oxygen ratio in hydrogen peroxide?
Review
- State the law of multiple proportions.
- In carbon dioxide, how many grams of oxygen would there be if there are 24 grams of carbon?
- How many grams of carbon would be present in carbon monoxide that contains 2.66 grams of oxygen?
Glossary
law of multiple proportions: Whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
Mass Ratio Calculation
Learning Objectives
- Define mass ratio.
- Calculate the mass ratio of an element in two different compounds.
What are the similarities and differences between these two equations?

One of the fundamental laws of chemistry deals with the fact that we cannot (using chemical means) create or destroy matter. When a reaction is run, the number of atoms of each specific type must be the same on both sides of the equation. For some materials, it turns out that one element can combine with a second element in more than one ratio. Carrying out mass ratio calculations helped establish the law of multiple proportions.
Copper reacts with chlorine to form two compounds. Compound A consists of 4.08 g of copper for every 2.28 g of chlorine. Compound B consists of 7.53 g of copper for every 8.40 g of chlorine. What is the lowest whole number mass ratio of copper that combines with a given mass of chlorine?
Step 1: List the known quantities and plan the problem.
- Known
- Compound A = 4.08 g Cu and 2.28 g Cl
- Compound B = 7.53 g Cu and 8.40 g Cl
Apply the law of multiple proportions to the two compounds. For each compound, find the grams of copper that combine with 1.00 g of chlorine by dividing the mass of copper by the mass of chlorine. Then find the ratio of the masses of copper in the two compounds by dividing the larger value by the smaller value.
Step 2: Calculate
- Compound A

- Compound B

Compare the masses of copper per gram of chlorine in the two samples.
![]()
The mass ratio of copper per gram of chlorine in the two compounds is 2:1.
Step 3: Think about your result.
The ratio is a small whole-number ratio. For a given mass of chlorine, compound A contains twice the mass of copper as does compound B.

Figure 6. CuCl2.
Summary
- The mass ratio gives the mass of an element that is found in combination with another element.
Practice
Use the link below to answer the following questions:
http://sciencing.com/calculate-mass-ratio-8326233.html
- What is the mass ratio?
- What is the hydrogen:water mass ratio?
- How many molecules of water per molecule of oxygen?
Review
- What does the mass ratio tell us?
- In the compound CH4, what is the carbon:hydrogen mass ratio?
- Methane is CH4 and ethane is C2H6. What is the mass ratio of carbon per gram of hydrogen in the two compounds?
Dalton’s Atomic Theory
Learning Objectives
List the components of Dalton’s atomic theory.
philosophers and Science

Pick a little, talk a little, pick a little, talk a little,
Cheep cheep cheep, talk a lot, pick a little more
These lyrics from the musical “Music Man” summed up the way science was done for centuries. OK, the lyrics referred to a group of gossiping ladies, but the outcome was the same.
The Greek and Roman philosophers debated, discussed, and sometimes even attacked one another. But the mode of discovery was talk. There was no experimentation – the idea had not been thought of yet. So science did not develop very far and there was no reliable way to establish what was true and what was false.
John Dalton

Figure 7. Dalton.
While it must be assumed that many more scientists, philosophers and others studied the composition of matter after Democritus, a major leap forward in our understanding of the composition of matter took place in the 1800s with the work of the British scientist John Dalton. He started teaching school at age twelve, and was primarily known as a teacher. In his twenties, he moved to the growing city of Manchester, where he was able to pursue some scientific studies. His work in several areas of science brought him a number of honors. When he died, over 40,000 people in Manchester marched at his funeral.
Dalton studied the weights of various elements and compounds. He noticed that matter always combined in fixed ratios based on weight, or volume in the case of gases. Chemical compounds always contain the same proportion of elements by mass, regardless of amount, which provided further support for Proust’s law of definite proportions. Dalton also observed that there could be more than one combination of two elements.
Dalton’s Atomic Theory (1804)
From his experiments and observations, as well as the work from peers of his time, Dalton proposed a new theory of the atom. This later became known as Dalton’s atomic theory. The general tenets of this theory were as follows:
- All matter is composed of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements can combine in simple whole number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated, or rearranged
Dalton’s atomic theory has been largely accepted by the scientific community, with the exception of three changes. We know now that (1) an atom can be further sub-divided, (2) all atoms of an element are not identical in mass, and (3) using nuclear fission and fusion techniques, we can create or destroy atoms by changing them into other atoms.

Figure 8. Dalton’s symbols.
Summary
- Dalton proposed his atomic theory in 1804.
- The general tenets of this theory were as follows:
- All matter is composed of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements can combine in simple whole number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated, or rearranged
Practice
Use the link below to do the exercise. Read the sections and take the quiz at the end.
http://antoine.frostburg.edu/chem/senese/101/atoms/dalton.shtml
Review
- How did the Greek and Roman philosophers study nature?
- When did John Dalton start teaching school?
- Did Dalton believe that atoms could be created or destroyed?
- List the basic components of Dalton’s atomic theory.
- What parts of the theory are not considered valid any more?
Glossary
- atom: The smallest unit of an element.
- atomic theory: The general tenets of Dalton’s atomic theory were as follows:
- All matter is composed of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties.
- Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements can combine in simple whole number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated, or rearranged
Cathode Ray Tube
Learning Objectives
- Describe how the Crooke’s tube functioned.
- Describe experiments that showed that cathode rays had mass.
How old do you think this TV is?
 The TV set seen above is becoming harder and harder to find these days. The main reason is because they are older and based on outdated technology. The new TV sets are flat screen technology that take up less space and give better picture quality, especially with the advent of high-definition broadcasting.
The TV set seen above is becoming harder and harder to find these days. The main reason is because they are older and based on outdated technology. The new TV sets are flat screen technology that take up less space and give better picture quality, especially with the advent of high-definition broadcasting.
The technology used in the older TV sets used cathode ray tubes. A beam of electrons was sprayed to a picture tube which was treated to react with the electrons to produce an image. Similar CRT devices were used in computer monitors, now also replaced by flat screen monitors.
Discovery of the Electron
The first discovery of a subatomic particle was a result of experiments into the nature of the relationship between electricity and matter.
Cathode Rays
The first cathode ray tube prototype was developed by Heinrich Geissler, a German glassblower and physicist. He used a mercury pump to create a vacuum in a tube. Geissler explored a number of techniques to remove air from the tube and to prevent leaks, as well as ways to get good connections of the wires in the tubes.
In 1878, Sir William Crookes, a British scientist, displayed the first cathode rays using a modification of the Geissler apparatus. His major contribution to construction of the tube was to develop ways to evacuate almost all the air from the tube. Crookes also carried out many experiments using more reliable equipment to confirm earlier finding about the properties of cathode rays. He discovered two things which supported the hypothesis that the cathode ray consisted of a stream of particles:
- When an object was placed between the cathode and the opposite end of the tube, it cast a shadow on the glass. The shadow caused by the object indicates that particles were being blocked on their way from the cathode to the anode.

- A cathode ray tube was constructed with a small metal rail between the two electrodes. Attached to the rail was a paddle wheel capable of rotating along the rail. Upon starting up the cathode ray tube, the wheel rotated from the cathode towards the anode. Notice that the cathode and anode are positioned so that the rays will strike the top of the paddle wheel. Crookes concluded that the cathode ray was made of particles which must have mass.

Figure 9. The cathode ray tube was first invented by Sir William Crookes.
Further Research with the Crookes Tube
Crookes’ work opened the door to a number of important discoveries. Other scientists were able to demonstrate that the “cathode ray” was actually a stream of electrons . In 1897, Karl Ferdinand Braun developed the first oscilloscope, using a cathode ray tube to see an electrical pulse as it passed through the instrument. The invention of television would not have been possible without the cathode ray tube. Work with a modified system led to the discovery of X-rays in 1895 by the German physicist Wilhelm Roentgen. This simple device has led to major advances in science and technology.
Summary
- The cathode ray tube was first invented by Sir William Crookes.
- Experiments showed that the rays had mass.
Practice
Use the link below to answer the following questions:
http://www.madehow.com/inventorbios/92/William-Crookes.html
- What did Crookes start to study in college?
- Who changed his mind and what did he then focus on?
- What element did Crookes discover?
- What did Crookes think was happening in the tube?
Review
- Who developed the first cathode ray tube?
- What improvement did Crookes make to the cathode ray tube?
- How did Crookes show there were particles being emitted?
- What did Karl Ferdinand Braun invent?
- What did Wilhelm Roentgen invent?
Glossary
- Crookes: In 1878, Sir William Crookes, a British scientist, displayed the first cathode rays using a modification of the Geissler apparatus. His major contribution to construction of the tube was to develop ways to evacuate almost all the air from the tube.
- cathode ray: A stream of electrons.
- electron: A subatomic particle with a negative charge.
Electrons
Learning Objectives
Describe evidence from cathode ray tube experiments that demonstrate properties of the electron.
What causes a power outage?

In a power outage all your electrical equipment suddenly stops working. The radio was on just a minute ago and now it is silent. What happened? Somewhere between a power generator and your electrical device was an interruption. Power stopped flowing through the wires and into your radio. That “power” turns out to be electrons that move through the wires and cause an electrical current to flow.
Is There Anything Inside an Atom?
As the nineteenth century began to draw to a close, the concept of atoms was well-established. We could determine the mass of different atoms and had some good ideas about the atomic composition of many compounds. Dalton’s atomic theory held that atoms were indivisible, so scientists did not ask questions about what was inside the atom – it was solid and could not be broken down further. But then things began to change.
The Electron
In 1897, English physicist J.J. Thomson (1856-1940) experimented with a device called a cathode ray tube, in which an electric current was passed through gases at low pressure. A cathode ray tube consists of a sealed glass tube fitted at both ends with metal disks called electrodes. The electrodes are then connected to a source of electricity. One electrode, called the anode , becomes positively charged while the other electrode, called the cathode , becomes negatively charged. A glowing beam (the cathode ray) travels from the cathode to the anode.

Earlier investigations by Sir William Crookes and others had been carried out to determine the nature of the cathode ray. Thomson modified and extended these experiments in an effort to learn about these mysterious rays. He discovered two things, which supported the hypothesis that the cathode ray consisted of a stream of particles.
- When an object was placed between the cathode and the opposite end of the tube, it cast a shadow on the glass.
- A cathode ray tube was constructed with a small metal rail between the two electrodes. Attached to the rail was a paddle wheel capable of rotating along the rail. Upon starting up the cathode ray tube, the wheel rotated from the cathode towards the anode. This proved that the cathode ray was made of particles which must have mass. Crooke had first observed this phenomenon and attributed it to pressure by these particles on the wheel. Thomson correctly surmised that these particles were producing heat, which caused the wheel to turn.
In order to determine if the cathode ray consisted of charged particles, Thomson used magnets and charged plates to deflect the cathode ray. He observed that cathode rays were deflected by a magnetic field in the same manner as a wire carrying an electric current, which was known to be negatively charged. In addition, the cathode ray was deflected away from a negatively charged metal plate and towards a positively charged plate.

Thomson knew that opposite charges attract one another, while like charges repel one another. Together, the results of the cathode ray tube experiments showed that cathode rays are actually streams of tiny negatively charged particles moving at very high speeds. While Thomson originally called these particles corpuscles, they were later named electrons.
Thomson conducted further experiments, which allowed him to calculate the charge-to-mass ratio ![]() of the electron. In units of coulombs to grams, this value is 1.8 × 10 8 Coulombs/gram. He found that this value was a constant and did not depend on the gas used in the cathode ray tube or on the metal used as the electrodes. He concluded that electrons were negatively charged subatomic particles present in atoms of all elements.
of the electron. In units of coulombs to grams, this value is 1.8 × 10 8 Coulombs/gram. He found that this value was a constant and did not depend on the gas used in the cathode ray tube or on the metal used as the electrodes. He concluded that electrons were negatively charged subatomic particles present in atoms of all elements.
Watch a video of a cathode ray tube experiment:
www.dlt.ncssm.edu/core/Chapter3-Atomic_Str_Part1/cathode-rm-lg.htm .
Summary
- Cathode rays are deflected by a magnetic field.
- The rays are deflected away from a negatively charged electrical field and toward a positively charge field.
- The charge/mass ratio for the electron is 1.8 × 10 8 Coulombs/gram.
Practice
Use the link below to answer the following questions:
http://www.nobelprize.org/nobel_prizes/physics/laureates/1906/thomson-bio.html
- How old was Thomson when he enrolled in college?
- What was his academic position at Cambridge?
- Where and when did he announce his discovery of the electron?
- What was he awarded in 1906?
Review
- What is electric power made up of?
- Whose work did Thomson repeat and revise?
- What experiment did Thomson perform that showed cathode rays to be particles?
- How did he show that these particles had a charge on them?
- Was the charge positive or negative?
Glossary
- anode: Positively charged electrode, when electric current runs through a cathode ray tube.
- cathode : Negatively charged electrode, when electric current runs through a cathode ray tube.
- magnetic field: An area where magnetism acts. It deflects cathode rays.
Oil Drop Experiment
Learning Objectives
Describe the oil drop experiment.
How tall are you? How much do you weigh? Questions like these are easy to answer because we have the tools to make the measurements. A yard stick or tape measure will suffice to measure height. You can stand on a bathroom scale and determine your weight.
But it is a very different matter to measure properties of objects that we cannot see with the naked eye. If we want to measure the size of a germ, we have to use a microscope. To learn the size of a single molecule, we have to use even more sophisticated instruments. So how would we measure something even smaller than a molecule, even smaller than an atom?
Charge and Mass of the Electron

Figure 10. Robert Millikan.
The man who measured properties of the electron was Robert Millikan (1868–1953). He taught himself physics while a student at Oberlin College since there was nobody on the faculty to instruct him in this field. Millikan completed postgraduate research training in the U.S. and in Germany. His studies on the properties of the electron proved to be of great value in many areas of physics and chemistry.
Oil Drop Experiment
Millikan carried out a series of experiments between 1908 and 1917 that allowed him to determine the charge of a single electron, famously know as the oil drop experiment.
He sprayed tiny drops of oil into a chamber. In his first experiment, he simply measured how fast the drops fell under the force of gravity. He could then calculate the mass of the individual drops. Then he sprayed oil drops and applied an electrical charge to them by shining X-rays up through the bottom of the apparatus. The X-rays ionized the air, causing electrons to attach to the oil drops. The oil drops picked up static charge and were suspended between two charged plates. Millikan was able to observe the motion of the oil drops with a microscope and found that the drops lined up in a specific way between the plates, based on the number of electric charges they had acquired.

Figure 11. Oil drop experiment.
Millikan used the information to calculate the charge of an electron. He determined the charge to be 1.5924 × 10 –19 C, where C stands for coulomb , which is one ampere/second. Today the accepted value of the charge of an electron is 1.602176487 × 10 –19 C. Millikan’s experimental value proved very accurate; it is within 1% of the currently accepted value. Millikan later used the information from his oil drop experiment to calculate the mass of an electron. The accepted value today is 9.10938215 ×10 –31 kg. The incredibly small mass of the electron was found to be approximately 1/1840 the mass of a hydrogen atom. Therefore, scientists realized that atoms must contain another particle that carries a positive charge and is far more massive than the electron.
Summary
- The oil drop experiment allowed Millikan to determine the charge on the electron.
- He later used this data to determine the mass of the electron.
Practice
Use the link below to answer the following questions:
http://www.aip.org/history/gap/Millikan/Millikan.html
- Why did he take a position at Chicago?
- How did he first make his mark at Chicago?
- Was he happy with his situation? Explain your answer.
- Why did Millikan use oil drops instead of water?
- What other contributions did Millikan make to science?
Review
- How did Millikan learn physics in college?
- What did Millikan use to pick up static charge?
- Where did the oil drops go to be measured?
Glossary
- coulomb: C is short for coulomb, which is one ampere/second.
- electron: A subatomic particle with a negative charge.
Protons
Learning Objectives
Describe Goldstein’s research that led to the discovery of the proton.
Can you name this car?

Describing what we can see is a fairly easy matter. If we are asked to describe the sports car illustrated below, we could all quickly come up with a fairly accurate description. A person knowledgeable about cars would include more details, but everyone would have the basic information in their description.
What makes the description easy to come up with? We can see it, we have a common language to describe it (size, color, construction), and we have a basic idea of what it is (a car, not a house or a tree). Scientists have far more difficulty in describing things they cannot see. There is no way to look directly at an atom and see its detailed structure. When a discovery is first made, there is often no language to use to tell others exactly what it is. This was the problem in talking about the atom and its structure.
Putting the Puzzle Pieces Together
Research builds upon itself – one piece connects to another. Sometimes the puzzle doesn’t seem to make sense because some of the pieces are missing at the moment. Each finding gives a clearer picture of the whole and also raises new questions. The detective work that led to the discovery of the proton was built upon finding pieces to the puzzle and putting them together in the right way.
The electron was discovered using a cathode ray tube. An electric current was passed from the cathode (the negative pole) to the anode (positive pole). Several experiments showed that particles were emitted at the cathode and that these particles had a negative charge. These experiments demonstrated the presence of electrons.

Figure 12. JJ Thomson’s experiment with cathode rays.
If cathode rays are electrons that are given off by the metal atoms of the cathode, then what remains of the atoms that have lost those electrons? We know several basic things about electrical charges. They are carried by particles of matter. Millikan’s experiment showed that they exist as whole-number multiples of a single basic unit. Atoms have no overall electrical charge, meaning that each and every atom contains an exactly equal number of positively and negatively charged particles. A hydrogen atom is the simplest kind of atom with only one electron. When that electron is removed, a positively charged particle should remain.
Discovery of the Proton
In 1886 Eugene Goldstein (1850–1930) discovered evidence for the existence of this positively charged particle. Using a cathode ray tube with holes in the cathode, he noticed that there were rays traveling in the opposite direction from the cathode rays. He called these canal rays and showed that they were composed of positively charged particles. The proton is the positively charged subatomic particle present in all atoms. The mass of the proton is about 1840 times the mass of the electron.
Summary
- When an electron is removed from a hydrogen atom, a proton remains.
- Goldstein observed rays travelling in the opposite direction of the cathode rays in a cathode ray tube.
- He demonstrated that these rays were positive particles and called the canal rays.
Practice
Use the link below to answer the following questions:
- What color is produced by electrons in the cathode ray tube?
- What color is produced by protons in the cathode ray tube?
- Could the “canal rays” be deflected by a magnetic field?
Review
- Why is it easy to describe things we can see?
- What are some problems in describing new ideas in science?
- Why did researchers believe that the particle left after electrons were emitted had to be positive?
- How do we know the proton has a positive charge?
- How many electrons does it take to weight the same as one proton?
Glossary
- canal ray: Using a cathode ray tube with holes in the cathode, he noticed that there were rays traveling in the opposite direction from the cathode rays. Eugene Goldstein (1850–1930) called these canal rays and showed that they were composed of positively charged particles.
- cathode: Negatively charged electrode, when electric current runs through a cathode ray tube.
- proton: The positively charged subatomic particle present in all atoms.
Neutrons
Learning Objectives
- Describe research findings that led to the discovery of the neutron.
- Describe uses for neutrons.
Was Sherlock Holmes real or the product of someone’s imagination?

The most famous detective in history and literature never existed. Sherlock Holmes was the creation of the British author Sir Arthur Conan Doyle. This mythical person had capabilities far beyond those of mere mortals. Holmes was capable of spotting the tiniest clue, the smallest piece of evidence to solve the crime. He could link all sorts of seemingly irrelevant data into a coherent whole to clear up whatever mystery he was dealing with.
The Quest for the Neutron
Clues are generally considered to involve the presence of something—a footprint, a piece of fabric, a bloodstain, something tangible that we can measure directly. The discoveries of the electron and the proton were accomplished with the help of those kinds of clues. Cathode ray tube experiments showed both the negatively charged electrons emitted by the cathode and the positively charged proton (also emitted by the cathode). The neutron was initially found not by a direct observation, but by noting what was not found.
Research had shown the properties of the electron and the proton. Scientists learned that approximately 1837 electrons weighed the same as one proton. There was evidence to suggest that electrons went around the heavy nucleus composed of protons. Charge was balanced with equal numbers of electrons and protons which made up an electrically neutral atom. But there was a problem with this model—the atomic number (number of protons) did not match the atomic weight. In fact, the atomic number was usually about half the atomic weight. This indicated that something else must be present. That something must weigh about the same as a proton, but could not have a charge – this new particle had to be electrically neutral.
In 1920 Ernest Rutherford tried to explain this phenomenon. He proposed that the “extra” particles were combinations of protons and electrons in the nucleus. These new particles would have a mass very similar to a proton, but would be electrically neutral since the positive charge of the proton and the negative charge of the electron would cancel each other out.
In 1930 German researchers bombarded the element beryllium with alpha particles (helium nuclei containing two protons and two neutrons with a charge of +2). The particles produced in this process had strong penetrating power, which suggested they were fairly large. In addition, they were not affected by a magnetic field, so they were electrically neutral. The French husband-wife research team of Frederic and Irene Joliot-Curie used these new “rays” to bombard paraffin, which was rich in protons. The unknown particles produced a large emission of protons from the paraffin.
The English physicist James Chadwick (1891–1974) repeated these experiments and studied the energy of these particles. By measuring velocities, he was able to show that the new particle has essentially the same mass as a proton. So we now have a third subatomic particle with a mass equal to that of a proton, but with no charge. This particle is called the neutron. Chadwick won the Nobel Prize in Physics in 1935 for his research.
Neutron Applications
Neutrons can be used in a variety of ways. One important use is in nuclear fission to produce new isotopes. A neutron will collide with a large atom (such as uranium) and cause it to split into smaller atoms, such as in the Figure 13.

Figure 13. A neutron collides with a large atom, splitting it into smaller atoms.
Nuclear reactors utilize chain reactions involving neutrons to heat water which drive turbines for the generation of electricity. When a neutron collides with a large atom, the atom splits with the release of more neutrons and also a large amount of energy. The energy converts water to steam for the turbine, while the neutrons serve to continue the chain reaction (see Figure 14).

Figure 14. How nuclear fission produces new isotopes.
Summary
- Rutherford proposed that “extra” particles in nucleus were combinations of protons and electrons.
- Bombardment of beryllium with alpha particles produced large, neutral particles.
- Chadwick determined the mass of the neutron.
- Nuclear fission produces new elements.
- Nuclear reactors use chain reactions to produce heat.
Practice
Read this page on the neutron to answer the following questions:
- When did Rutherford first propose the existence of the neutron?
- Why are protons poorly penetrating?
- Who did Chadwick do research with?
- Why did the Curies believe that gamma rays were not responsible for proton release?
- How did Chadwick detect the production of protons?
Review
- What is a clue?
- What clues suggested the existence of the neutron?
- How did Rutherford try to explain the differences between the number of protons in the nucleus and the atomic weight?
- What did German researchers find when they bombarded beryllium with alpha particles?
- Describe the research of the Curies.
- How did Chadwick determine these new particle were about the same size as the proton?
Glossary
- neutron: A subatomic particle with no electric charge.
- fission: Neutrons are used to produce new isotopes. A neutron will collide with a large atom and cause it to split into smaller atoms.
- chain reaction: When a neutron collides with a large atom, the atom splits with the release of more neutrons and also a large amount of energy. The neutrons then continue the chain reaction. Nuclear reactors use chain reactions to produce heat.
Thomson’s Atomic Model
Learning Objectives
- Explain what a model is.
- Describe the “plum pudding” model of the atom.
What is this model airplane composed of?
 Millions of children over the years have enjoyed building models—this model airplane is one example of the types of models that can be constructed. Perhaps sixty years ago the models were made of balsa wood, a very light material. Parts would be cut by hand, carefully glued together, and then covered with paper or other fabric.
Millions of children over the years have enjoyed building models—this model airplane is one example of the types of models that can be constructed. Perhaps sixty years ago the models were made of balsa wood, a very light material. Parts would be cut by hand, carefully glued together, and then covered with paper or other fabric.
The development of plastics made the construction of model aircraft much simpler in many respects. And, the end-product is more durable and damage-proof.
A model serves a useful purpose—it gives us an idea of what the real thing is like. The model plane seen above has wings, a tail, and an engine just like the real thing. This model also has a propeller, as is the case with most small planes and some smaller passenger planes. However, the model is not the real thing. We certainly cannot fly people or cargo in the model (besides maybe a tiny mouse), but we can get some idea of what a real plane looks like and how it works.
Science uses many models to explain ideas. We model the electron as a very small particle with a negative charge. That gives us a picture, but a very incomplete one. This picture works fine for most chemists, but is inadequate for a physicist. Models give us a start toward understanding structures and processes, but certainly are not a complete representation of the entity we are examining.
Atomic Models
The electron was discovered by J.J. Thomson in 1897. The existence of protons was also known, as was the fact that atoms were neutral in charge. Since the intact atom had no net charge and the electron and proton had opposite charges, the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom. This is a difficult task because of the incredibly small size of the atom. Therefore, scientists set out to design a model of what they believed the atom could look like. The goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.
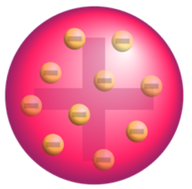
Figure 15. The “plum pudding” model.
Following the discovery of the electron, J.J. Thomson developed what became known as the “ plum pudding ” model in 1904. Plum pudding is an English dessert similar to a blueberry muffin. In Thomson’s plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge like blueberries stuck into a muffin. The positive matter was thought to be jelly- like or a thick soup. The electrons were somewhat mobile. As they got closer to the outer portion of the atom, the positive charge in the region was greater than the neighboring negative charges and the electron would be pulled back more toward the center region of the atom.
However, this model of the atom soon gave way to a new model developed by New Zealander Ernest Rutherford (1871-1937) about five years later. Thomson did still receive many honors during his lifetime, including being awarded the Nobel Prize in Physics in 1906 and a knighthood in 1908.
Summary
- A model gives an idea of what something looks like, but is not the real thing.
- The “plum pudding” model of the atom consisted of a uniform sphere of positive charge with negative electrons embedded in the sphere.
Practice
Use the link below to answer the following questions:
http://www.universetoday.com/38326/plum-pudding-model/
- In the plum pudding model of the atom, what are the plums?
- In this model, what is the dough?
- What was the major purpose of the plum pudding model?
- How is this model different from modern modes of the atom?
Review
- What is a model?
- Why are models useful in science?
- In Thomson’s model of the atom, where were the electrons?
- What was the positive charge in this model?
- What kept the electrons in the atom?
- Whose model replaced Thomson’s?
- What awards did Thomson receive?
Glossary
- atomic model: When scientists set out to design a model of what they believed the atom could look like, the goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.
- plum pudding : In 1904 J.J. Thomson developed this model. The electrons were stuck into a uniform lump of positive charge like blueberries in a muffin. The positive matter was thought to be jelly- like or a thick soup. The electrons could move around somewhat. As they got closer to the outer portion of the atom, the positive charge in the region was greater than the neighboring negative charges and the electron would be pulled back more toward the center region of the atom.
Rutherford’s Atomic Model
Learning Objectives
- Describe Rutherford’s gold foil experiment.
- Describe the nuclear model of the atom.
How much space do bricks occupy?

As we look at the world around us, it looks pretty solid. We hit a wall with our hand and the hand stops – it does not (normally) go through the wall. We think of matter as occupying space. But there is a lot of empty space in matter. In fact, most of the matter is empty space.
The Gold Foil Experiment
In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. They bombarded very thin sheets of gold foil with fast moving alpha particles . Alpha particles, a type of natural radioactive particle, are positively charged particles with a mass about four times that of a hydrogen atom.

Figure 16. (A) The experimental setup for Rutherford’s gold foil experiment: A radioactive element that emitted alpha particles was directed toward a thin sheet of gold foil that was surrounded by a screen which would allow detection of the deflected particles. (B) According to the plum pudding model (top) all of the alpha particles should have passed through the gold foil with little or no deflection. Rutherford found that a small percentage of alpha particles were deflected at large angles, which could be explained by an atom with a very small, dense, positively-charged nucleus at its center (bottom).
According to the accepted atomic model, in which an atom’s mass and charge are uniformly distributed throughout the atom, the scientists expected that all of the alpha particles would pass through the gold foil with only a slight deflection or none at all. Surprisingly, while most of the alpha particles were indeed undeflected, a very small percentage (about 1 in 8000 particles) bounced off the gold foil at very large angles. Some were even redirected back toward the source. No prior knowledge had prepared them for this discovery. In a famous quote, Rutherford exclaimed that it was “as if you had fired a 15-inch [artillery] shell at a piece of tissue paper and it came back and hit you.”
Rutherford needed to come up with an entirely new model of the atom in order to explain his results. Because the vast majority of the alpha particles had passed through the gold, he reasoned that most of the atom was empty space. In contrast, the particles that were highly deflected must have experienced a tremendously powerful force within the atom. He concluded that all of the positive charge and the majority of the mass of the atom must be concentrated in a very small space in the atom’s interior, which he called the nucleus. The nucleus is the tiny, dense, central core of the atom and is composed of protons and neutrons.
Rutherford’s atomic model became known as the nuclear model . In the nuclear atom, the protons and neutrons, which comprise nearly all of the mass of the atom, are located in the nucleus at the center of the atom. The electrons are distributed around the nucleus and occupy most of the volume of the atom. It is worth emphasizing just how small the nucleus is compared to the rest of the atom. If we could blow up an atom to be the size of a large professional football stadium, the nucleus would be about the size of a marble.
Rutherford’s model proved to be an important step towards a full understanding of the atom. However, it did not completely address the nature of the electrons and the way in which they occupied the vast space around the nucleus. It was not until some years later that a full understanding of the electron was achieved. This proved to be the key to understanding the chemical properties of elements.
Watch a video that explains the gold foil experiment:
Summary
- Bombardment of gold foil with alpha particles showed that some particles were deflected.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
Practice
Use the link below to answer the following questions:
http://www.icbse.com/topics/rutherfords-model-atom
- How thick was the gold foil?
- What alpha source did he use?
- How many were deflected straight back?
- What was one drawback of Rutherford’s theory?
Review
- When did Rutherford and coworkers carry out their research?
- What is an alpha particle?
- How did Rutherford explain the observation that most alpha particles went straight through the gold foil?
- What did he say about the particles that were deflected?
- Describe Rutherford’s nuclear model.
Glossary
- alpha particle: A type of natural radioactive particle, are positively charged particles with a mass about four times that of a hydrogen atom.
- nuclear model : The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
Atomic Nucleus
Learning Objectives
Describe the current model of the atomic nucleus.
How is science like a jigsaw puzzle?
 Many people enjoy putting jigsaw puzzles together. As the different pieces go together, the picture begins to become clearer. When the puzzle is completed, you see that what had been a confused collection of individual components that made little or no sense by themselves fit together to give a clear picture.
Many people enjoy putting jigsaw puzzles together. As the different pieces go together, the picture begins to become clearer. When the puzzle is completed, you see that what had been a confused collection of individual components that made little or no sense by themselves fit together to give a clear picture.
Science works the same way as a jigsaw puzzle. Different researchers make individual discoveries that answer a specific question or questions. When enough data are gathered, we get a better understanding of a process or a structure. The experiments of Crooke, Millikan, Rutherford, and many others gave us pieces of the puzzle that is the atomic nucleus.
Different Models of the Nucleus
After the electron and proton were discovered, people began to try to build a picture of the atom. The Bohr model had electrons mixed in with a positive core of some sort that provided electrical neutrality. Rutherford showed that this model was incomplete. His picture of the atom involved a small solid core that alpha particles could zoom past with very few collisions. So the picture of the atom became a little clearer – electrons surrounded a very small core nucleus. The discovery of the neutron helped fill out the picture even more. We now have protons and neutrons in a concentrated center of the atom with electrons surrounding the nucleus.
One problem still existed. We have a number of positively charged protons in the nucleus. Why don’t they push each other apart? Physicists postulate a strong nuclear force that acts at very short distances. At these distances the attraction between protons is greater than the force causing the positive charges to push each other away. Neutrons are also involved in this process somehow. So the strong force holds protons together, it holds neutrons together, and it causes protons and neutrons to be attracted to one another.
Summary
- Rutherford proposed a model of the atomic nucleus which had a solid core.
- There is no good explanation for why the nuclear particles stay together.
Practice
Use the link below to answer the following questions:
http://www.chemistryexplained.com/Ar-Bo/Atomic-Nucleus.html
- What is the radius of the nucleus as compared to the radius of the atom?
- What is the density of the nucleus?
Review
- What was our first model of the atom?
- How did Rutherford change our thinking about atomic structure?
- What is our current picture of the atom?
- Why is the presence of positively charged protons a problem with current models of the atom?
- How do we explain why the nucleus does not fall apart?
Glossary
- electron: A subatomic particle with a negative charge.
- neutron: A subatomic particle with no electric charge.
- proton: The positively charged subatomic particle present in all atoms.
- nucleus: The positively charged center of an atom, containing most of its mass.
Atomic Number
Learning Objectives
- Define the atomic number.
- Relate the number of electrons in an element to the atomic number for that element.
What is unique about each one of us?
 For the vast majority of people, it is not their name, because it is quite possible for others in the world to have the same name (check it out by doing an internet search for your name and see how many other of “you” there are). It is not your physical description. Eye-witnesses to crime scenes often pick the wrong person when trying to identify the criminal.
For the vast majority of people, it is not their name, because it is quite possible for others in the world to have the same name (check it out by doing an internet search for your name and see how many other of “you” there are). It is not your physical description. Eye-witnesses to crime scenes often pick the wrong person when trying to identify the criminal.
There may be some unique identifiers for us. If you have a cell phone in your name, nobody else in the world has that number. Email addresses are different for each of us, which is a good thing since we can email almost anywhere in the world.
Our DNA is unique, but getting a DNA analysis is expensive and time-consuming, so we really don’t want to have to explore that.
Organizing the Elements
One of the goals of science is to discover the order in the universe and to organize information that reflects that order. As information about the different elements was made known, efforts were made to see if there were patterns in all of the data. An early attempt to organize data was made by Mendeleev, who developed the first periodic table. His data set was based on atomic weights and was instrumental in providing clues as to the possible identity of new elements. Once we learned the details of the atomic nucleus, the table was based on the number of protons in the nucleus, called the atomic number of the element.
Atomic Number

Figure 17. How can you determine the atomic number of an element?
The atomic number (Z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. Elements are different because of their atomic number. The periodic table displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element’s atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Following on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, and so forth.
Since atoms are neutral, the number of electrons is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Manganese (atomic number 25) would have twenty-five protons and twenty-five electrons.

Figure 18. The periodic table classifies elements by atomic number.
The classification of elements by atomic number allows us to understand many properties of the atom and makes it possible to predict behaviors instead of just having to memorize everything.
Summary
- The atomic number (Z) of an element is the number of protons in the nucleus of each atom of that element
- The number of electrons is equal to the number of protons in an element.
Practice
Use the link below to answer the following questions:
http://en.citizendium.org/wiki/atomic_number
- What letter is used by convention to designate the atomic number?
- What determines the chemical properties of an element?
- What are the atomic numbers of the elements that appear in nature?
- How many elements were known in John Dalton’s day?
Review
- Name two unique identifiers of people.
- Who developed the first periodic table?
- What was this table based upon?
- What is the current periodic table based upon?
- What does the atomic number represent?
- How many protons are in the following elements:?
- Ne
- Ca
- Pt
- Write the symbol for the element with the following atomic number:
- 18
- 41
- 82
- 12
Glossary
- periodic table: This table displays all of the known elements and is arranged in order of increasing atomic number.
- atomic weight: Each chemical element has an atom with a given mass.
- atomic number : The number of protons in the nucleus of each atom of that element.
Mass Number
Learning Objectives
- Define mass number.
- Calculate the mass number when given number of protons and neutrons.
- Calculate number of neutrons when given atomic number.
How can you determine the mass of a chemical?

Often a student will need to weigh out a chemical for an experiment. If he or she uses a watch glass (a small, round piece that will hold the solid chemical), the weight of the watch glass must be determined first. Then the solid is added to the glass and the weight of the glass plus the solid is measured. The balance reading will be the total of the glass plus the chemical.
History of Atomic Weight Determinations

As a part of his research on atoms, John Dalton determined a number of atomic weights of elements in the early 1800s. Atomic weights were the basis for the periodic table that Mendeleev developed. Originally all atomic weights were based on a comparison to hydrogen, which has an atomic weight of one. After the discovery of the proton, scientists assumed that the weight of an atom was essentially that of the protons – electrons were known to contribute almost nothing to the atomic weight of the element.
This approach worked until we learned how to determine the number of protons in an element. We then saw that the atomic weight for an element was often twice the number of protons (or more). The discovery of the neutron provided the missing part of the picture. The atomic mass now known to be the sum of the protons and neutrons in the nucleus.
Mass Number
Rutherford showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number is defined as the total number of protons and neutrons in an atom. Consider Table below which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number | Protons | Neutrons | Electrons | Mass Number |
| Hydrogen | H | 1 | 1 | 0 | 1 | 1 |
| Helium | He | 2 | 2 | 2 | 2 | 4 |
| Lithium | Li | 3 | 3 | 4 | 3 | 7 |
| Beryllium | Be | 4 | 4 | 5 | 4 | 9 |
| Boron | B | 5 | 5 | 6 | 5 | 11 |
| Carbon | C | 6 | 6 | 6 | 6 | 12 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since 2 + 2 = 4, we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but further examination of the Table above will show that this is not the case. Lithium, for example has three protons and four neutrons, leaving it with a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
Number of neutrons = mass number − atomic number
Atoms of the element chromium (Cr) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
- 52 − 24 = 28 neutrons in a chromium atom
The composition of any atom can be illustrated with a shorthand notation using the atomic number and the mass number. Both are written before the chemical symbol, with the mass number written as a superscript and the atomic number written as a subscript. The chromium atom discussed above would be written as: ![]()
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The above atom would be written as chromium-52.
Summary
- The mass number is defined as the total number of protons and neutrons in an atom.
- The number of neutrons = mass number − atomic number.
Practice
Use the link below to answer the following questions:
http://education.jlab.org/qa/pen_number.html
- What data in the periodic table tells you the number of protons in an atom?
- How do you determine the number of neutrons in an atom?
- What is the mass number for an atom?
Review
- Who first determined atomic weights for elements?
- What were the original atomic weights based on?
- Why were calculations based on numbers of protons not valid for determining atomic weights?
- A tin atom has an atomic number of 50 and a mass number of 118. How many neutrons are present in this atom?
- What is the mass number of a cobalt atom that has 27 protons and 30 neutrons?
Glossary
- mass number: The total number of protons and neutrons in an atom.
Isotopes
Learning Objectives
- Define isotope.
- Calculate the number of neutrons in an isotope when given atomic number and atomic mass.
Are all the members of the football team shown above identical?

They are on the same team and are all known by the same team name, but there are individual differences among the players. We do not expect the kicker to be as big as the quarterback. The tight end is very likely to weigh less than the defensive tackle on the other side of the ball. They play as a unit, but they have different weights and heights.
The history of the atom is full of some of these differences. Although John Dalton stated in his atomic theory of 1804 that all atoms of an element are identical, the discovery of the neutron began to show that this assumption was not correct. The study of radioactive materials (elements that spontaneously give off particles to form new elements) by Frederick Soddy (1877–1956) gave important clues about the internal structure of atoms. His work showed that some substances with different radioactive properties and different atomic weights were in fact the same element. He coined the term isotope from the Greek roots isos (íσος “equal”) and topos (τóπος “place”). He described isotopes as, “Put colloquially, their atoms have identical outsides but different insides.” Soddy won the Nobel Prize in Chemistry in 1921 for his work.
As stated earlier, not all atoms of a given element are identical. Specifically, the number of neutrons can be variable for many elements. As an example, naturally occurring carbon exists in three forms. Each carbon atom has the same number of protons (6), which is its atomic number. Each carbon atom also contains six electrons in order to maintain electrical neutrality. However the number of neutrons varies as six, seven, or eight. Isotopes are atoms that have the same number atomic number, but different mass numbers due to a change in the number of neutrons.
The three isotopes of carbon can be referred to as carbon-12 ![]() , carbon-13
, carbon-13 ![]() , and carbon-14
, and carbon-14 ![]() . Most elements naturally consist of mixtures of isotopes. Carbon has three natural isotopes, while some heavier elements can have many more. Tin has ten stable isotopes, the most of any element. The term nuclide refers to the nucleus of a given isotope of an element. A carbon atom is one of three different nuclides.
. Most elements naturally consist of mixtures of isotopes. Carbon has three natural isotopes, while some heavier elements can have many more. Tin has ten stable isotopes, the most of any element. The term nuclide refers to the nucleus of a given isotope of an element. A carbon atom is one of three different nuclides.
While the presence of isotopes affects the mass of an atom, it does not affect its chemical reactivity. Chemical behavior is governed by the number of electrons and the number of protons. Carbon-13 behaves chemically in exactly the same way as the more plentiful carbon-12.
Summary
- Isotopes are atoms that have the same atomic number, but different mass numbers due to a change in the number of neutrons.
- The term nuclide refers to the nucleus of a given isotope of an element.
- The atomic mass of an atom equals the sum of the protons and the neutrons.
Practice
Use the link below to answer the following questions:
http://www.ehow.com/about_4678737_discovered-isotope.html
- When was the term “isotope” first used?
- Are isotopes of an element chemically the same or different?
- How are radioactive isotopes used?
Review
- What does the term “isotope” mean
- Define “isotope.”
- What affects the chemical behavior of an atom?
- An isotope of yttrium has 39 protons and 59 neutrons. What is the atomic mass of that isotope?
- An isotope with an atomic mass of 193 has 116 neutrons. What is the atomic number of this isotope?
- An isotope of barium (atomic number 56) has an atomic mass of 138. How many neutrons are in the nucleus of this isotope?
Glossary
- isotope: Atoms that have the same number atomic number, but different mass numbers due to a change in the number of neutrons.
- nuclide: The nucleus of a given isotope of an element. A carbon atom is one of three different nuclides.
Atomic Mass Unit
Learning Objectives
- Define atomic mass unit.
- Explain how atomic mass units are determined.
How many gallons of gas can fill a car’s tank?
 The current system of measurement in the Unites States is a hodge-podge of different units, many of which are hard to interconvert, unlike the metric system. We at least have standardized units these days, unlike centuries past. At one time, measurements of length often were defined as the distance from the end of one appendage to another. For example, the yard would be defined as the distance from the king’s nose to the tip of his thumb when his arm was stretched out.
The current system of measurement in the Unites States is a hodge-podge of different units, many of which are hard to interconvert, unlike the metric system. We at least have standardized units these days, unlike centuries past. At one time, measurements of length often were defined as the distance from the end of one appendage to another. For example, the yard would be defined as the distance from the king’s nose to the tip of his thumb when his arm was stretched out.
Standardized measurements make it possible for people everywhere to get the same amount of something. Note the red and white labels on the gas pump above. These labels certify that the gas pump has been checked and is pumping an accurate gallon of gas. Standard measurements in science are very important so that we can compare experimental data from one lab to another and make sure we all are talking about the same thing.
Atomic Mass
Masses of individual atoms are very, very small. Using a modern device called a mass spectrometer, it is possible to measure such minuscule masses. An atom of oxygen-16, for example, has a mass of 2.66 × 10 -23 g. While comparisons of masses measured in grams would have some usefulness, it is far more practical to have a system that will allow us to more easily compare relative atomic masses. Scientists decided on using the carbon-12 nuclide as the reference standard by which all other masses would be compared. By definition, one atom of carbon-12 is assigned a mass of 12 atomic mass units (amu). An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. For example, one atom of helium-4 has a mass of 4.0026 amu. An atom of sulfur-32 has a mass of 31.972 amu.

Figure 19. Mass spectrometer schematic.
The carbon-12 atom has six protons and six neutrons in its nucleus for a mass number of 12. Since the nucleus accounts for nearly all of the mass of the atom, a single proton or single neutron has a mass of approximately 1 amu. However, as seen by the helium and sulfur examples, the masses of individual atoms are not whole numbers. This is because an atom’s mass is affected very slightly by the interactions of the various particles within the nucleus, and the small mass of the electron is taken into account.
Summary
- Carbon-12 is the reference for all atomic mass calculations.
- An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12.
- The mass of an atom is affected by the interactions of particles within the nucleus.
Practice
Use the link below to answer the following questions:
http://www.wisegeek.com/what-is-the-atomic-mass-unit.htm#lbss
- What is the atomic mass unit based on?
- What is another term for atomic mass unit?
- What mistake is in the second paragraph?
Review
- What instrument is used to measure the mass of atoms?
- How much does a single oxygen-16 atom weigh in grams?
- What is the reference standard for atomic mass units?
- How is an atomic mass unit defined?
- Why are the numbers for atomic mass of individual atoms not whole numbers?
Glossary
atomic mass unit: Abbreviated as “amu.” A mass equal to one twelfth the mass of an atom of carbon-12.
Calculating Atomic Mass
Learning Objectives
- Define atomic mass.
- Calculate atomic mass given relevant information about the isotopes.
Have you ever tried to move a boulder?
 You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop.
You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop.
The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job.
Most elements occur naturally as a mixture of two or more isotopes. Table below shows the natural isotopes of several elements, along with the percent natural abundance of each.
| Element | Isotope
(symbol) |
Percent natural
abundance |
Atomic
mass (amu) |
Average
atomic mass (amu) |
| Hydrogen |
|
99.985
0.015 negligible |
1.0078
2.0141 3.0160 |
1.0079 |
| Carbon |
|
98.89
1.11 trace |
12.000
13.003 14.003 |
12.011 |
| Oxygen |
|
99.759
0.037 0.204 |
15.995
16.995 17.999 |
15.999 |
| Chlorine |
|
75.77
24.23 |
34.969
36.966 |
35.453 |
| Copper |
|
69.17
30.83 |
62.930
64.928 |
63.546 |
For some elements, one particular isotope predominates greatly over the other isotopes. Naturally occurring hydrogen is nearly all hydrogen-1 and naturally occurring oxygen is nearly all oxygen-16. For many other elements, however, more than one isotope may exist in more substantial quantities. Chlorine (atomic number 17) is a yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses , you would get 36.
![]()
Clearly the actual average atomic mass from the last column of the table is significantly lower. Why? We need to take into account the percent natural abundances of each isotope in order to calculate what is called the weighted average. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. The sample problem below demonstrates how to calculate the atomic mass of chlorine.
SAMPLE PROBLEM: CALCULATING ATOMIC MASS
Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine.
Step 1: List the known and unknown quantities and plan the problem.
Known
- chlorine-35: atomic mass = 34.969 amu and % abundance = 75.77%
- chlorine-37: atomic mass = 36.966 amu and % abundance = 24.23%
Unknown
- Average atomic mass of chlorine
Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Step 2: Calculate

Note: Applying significant figure rules results in the 35.45 amu result without excessive rounding error. In one step:
![]()
Step 3: Think about your result.
The calculated average atomic mass is closer to 35 than to 37 because a greater percentage of naturally occurring chlorine atoms have the mass number of 35. It agrees with the value from the Table above .
Watch these videos to learn more about these calculations:
http://www.kentchemistry.com/links/AtomicStructure/atomicmasscalc.htm
Summary
- The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
- Calculations of atomic mass use the percent abundance of each isotope.
Review
- Define atomic mass.
- What information do you need to calculate atomic mass for an element?
- Calculate the atomic mass for carbon using the data provided in Table below .
| mass number | exact weight | percent abundance |
| 12 | 12.000000 | 98.90 |
| 13 | 13.003355 | 1.10 |
Glossary
- atomic mass: The weighted average of the atomic masses of the naturally occurring isotopes of that element.
- percent abundance: To calculate the weighted average, take into account the percent natural abundances of each isotope. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
Candela Citations
- Chemistry Concepts Intermediate. Authored by: Calbreath, Baxter, et al.. Provided by: CK12.org. Located at: http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike