Early History of the Periodic Table
Learning Objectives
- Describe Dobereiner’s triad approach to organizing elements.
- Describe Newland’s Law of Octaves.
When you go to the library to find a book, how do you locate it?
 If it is a fiction book, you look by author since the fiction materials are filed by the author’s last name. If you are looking for a non-fiction publication, you look in a catalog (most likely on a computer these days). The book you are looking for will have a number by the title. This number refers to the Dewey Decimal system, developed by Melvil Dewey in 1876 and used in over 200,000 libraries throughout the world.
If it is a fiction book, you look by author since the fiction materials are filed by the author’s last name. If you are looking for a non-fiction publication, you look in a catalog (most likely on a computer these days). The book you are looking for will have a number by the title. This number refers to the Dewey Decimal system, developed by Melvil Dewey in 1876 and used in over 200,000 libraries throughout the world.
Another system in wide use is the Library of Congress approach, developed in the late 1800s-early 1900s to organize the materials in the federal Library of Congress. This method is one of the most widely used ways to organize libraries in the world. Both approaches organize information so that people can easily find what they are looking for. Chemistry information also needs to be organized so we can see patterns of properties in elements.
Early Attempts to Organize Elements
By the year 1700, only a handful of elements had been identified and isolated. Several of these, such as copper and lead, had been known since ancient times. As scientific methods improved, the rate of discovery dramatically increased. With the ever-increasing number of elements, chemists recognized that there may be some kind of systematic way to organize the elements. The question was: how?
A logical way to begin grouping elements together was by their chemical properties. In other words, putting elements in separate groups based on how they reacted with other elements. In 1829, a German chemist, Johann Dobereiner (1780-1849), placed various groups of three elements into groups called triads . One such triad was lithium, sodium, and potassium. Triads were based on both physical as well as chemical properties. Dobereiner found that the atomic masses of these three elements, as well as other triads, formed a pattern. When the atomic masses of lithium and potassium were averaged together ![]() , it was approximately equal to the atomic mass of sodium (22.99). These three elements also displayed similar chemical reactions, such as vigorously reacting with the members of another triad: chlorine, bromine, and iodine. While Dobereiner’s system would pave the way for future ideas, a limitation of the triad system was that not all of the known elements could be classified in this way.
, it was approximately equal to the atomic mass of sodium (22.99). These three elements also displayed similar chemical reactions, such as vigorously reacting with the members of another triad: chlorine, bromine, and iodine. While Dobereiner’s system would pave the way for future ideas, a limitation of the triad system was that not all of the known elements could be classified in this way.
English chemist John Newlands (1838-1898) ordered the elements in increasing order of atomic mass and noticed that every eighth element exhibited similar properties. He called this relationship the “Law of Octaves .” Unfortunately, there were some elements that were missing and the law did not seem to hold for elements that were heavier than calcium. Newlands’s work was largely ignored and even ridiculed by the scientific community in his day. It was not until years later that another more extensive periodic table effort would gain much greater acceptance and the pioneering work of John Newlands would be appreciated.

Summary
- Johann Dobereiner organized elements in groups called triads.
- John Newland proposed the “Law of Octaves” for organizing the elements.
Practice
Look at History of the Development of the Periodic Table of Elements to answer the following questions:
- Who discovered the first element through scientific inquiry and what was the element?
- Describe the triad approach to constructing a periodic table.
- What contribution did A.E.Beguyer de Chancourtois make to the development of the periodic table?
- Why did John Newlands call his approach the Law of Octaves?
Review
- List some elements known since ancient times?
- What properties were the basis of the triad system?
- Why did Dobereiner believe that lithium, sodium, and potassium belonged in a triad?
- What was a shortcoming of the triad system?
- How did Newlands arrange the element?
- What was a problem with the “Law of Octaves”?
Glossary
- triad: In 1829, a German chemist, Johann Dobereiner (1780-1849), placed various groups of three elements into groups called triads. One such triad was lithium, sodium, and potassium. Triads were based on both physical as well as chemical properties. Dobereiner found that the atomic masses of these three elements, as well as other triads, formed a pattern.
- octave: English chemist John Newlands (1838-1898) ordered the elements in increasing order of atomic mass and noticed that every eighth element exhibited similar properties. He called this relationship the “Law of Octaves.”
Mendeleev’s Periodic Table
Learning Objectives
- Describe Mendeleev’s organization of the periodic table.
- State predictions made possible by this table.
When you study for a test, how do you approach the task?

One useful way is to use flash cards. You write down the vocabulary words, the foreign language terms, the math formulas, the chemistry reactions – anything you want to learn. Then you sort these cards into categories, topics that go together. This organization of information helps you see patterns in the material so you can tie different ideas together and make better sense of them.
The periodic table was first built using a set of cards. With this strategy, Mendeleev could organize and rearrange material until patterns emerged.

Figure 1. Mendeleev.
In 1869, Russian chemist and teacher Dmitri Mendeleev (1836–1907) published a periodic table of the elements. The following year, German chemist Lothar Meyer independently published a very similar table. Mendeleev is generally given more credit than Meyer because his table was published first and because of several key insights that he made regarding the table.
Mendeleev was writing a chemistry textbook for his students and wanted to organize all of the known elements at that time according to their chemical properties. He famously organized the information for each element on to separate note cards that were then easy to rearrange as needed. He discovered that when he placed them in order of increasing atomic mass, certain similarities in chemical behavior repeated at regular intervals. This type of a repeating pattern is called “periodic.” A pendulum that swings back and forth in a given time interval is periodic, as is the movement of the moon around the Earth.

Figure 2. Mendeleev’s 1869 periodic table.
In Figure 2, atomic mass increases from top to bottom of vertical columns, with successive columns going left to right. As a result, elements that are in the same horizontal row are groups of elements that were known to exhibit similar chemical properties. One of Mendeleev’s insights is illustrated by the elements tellurium (Te) and iodine (I). Notice that tellurium is listed before iodine even though its atomic mass is higher. Mendeleev reversed the order because he knew that the properties of iodine were much more similar to those of fluorine (F), chlorine (Cl), and bromine (Br) than they were to oxygen (O), sulfur (S), and selenium (Se). He simply assumed that there was an error in the determination of one or both of the atomic masses. As we will see shortly, this turned out not to be the case, but Mendeleev was indeed correct to group these two elements as he did.
Notice that there are several places in the table that have no chemical symbol, but are instead labeled with a question mark. Between zinc (Zn) and arsenic (As) are two such missing elements. Mendeleev believed that elements with atomic masses of 68 and 70 would eventually be discovered and that they would fit chemically into each of those spaces. Listed in the Table below are other properties that Mendeleev predicted for the first of these two missing elements, which he called “eka-aluminum,” compared with the element gallium.
| Eka-Aluminum (Ea) | Gallium (Ga) | |
| Atomic mass | 68 amu | 69.9 amu |
| Melting point | Low | 30.15°C |
| Density | 5.9 g/cm 3 | 5.94 g/cm 3 |
| Formula of oxide | Ea 2 O 3 | Ga 2 O 3 |
The element gallium was discovered four years after the publication of Mendeleev’s table, and its properties matched up remarkably well with eka-aluminum, fitting into the table exactly where he had predicted. This was also the case with the element that followed gallium, which was named eventually named germanium.
Mendeleev’s periodic table gained wide acceptance with the scientific community and earned him credit as the discoverer of the periodic law. Element number 101, synthesized in 1955, is named mendelevium after the founder of the periodic table. It would, however, be several years after Mendeleev died before the several discrepancies with the atomic masses could be explained and before the reasons behind the repetition of chemical properties could be fully explained.
Summary
- Mendeleev published his periodic table in 1869.
- His organization of elements was based on atomic mass.
- Mendeleev’s periodic table made it possible to predict properties of elements that had not yet been discovered.
Practice
- Where was Mendeleev born?
- Where did he teach?
- What is one important thing about Mendeleev’s table?
- What other contributions to chemistry did Mendeleev make?
Review
- When did Mendeleev publish his periodic table?
- Who else came out with a periodic table at about the same time?
- Why was Mendeleev’s table considered to be superior?
- What element did Mendeleev predict to exist?
- What element was named after Mendeleev?
Glossary
- Mendelevium: Element number 101, synthesized in 1955, is named mendelevium after Dmitri Mendeleev (1836-1907), the founder of the periodic table.
Periodic Law
Learning Objectives
- State the periodic law.
- Describe the organization of the periodic table.
How are these items related to one another?

We have all enjoyed playing the game “Clue.” The object of the game is to obtain information about a murder – who did it, where they did it, and what was used as the murder weapon. As the game progresses, clues are obtained by each player and they then must assemble these clues into a guess as to the criminal. Individual pieces of information takes on broader significance when put together with other parts of the puzzle.
When Mendeleev put his periodic table together, nobody knew about the existence of the nucleus. It was not until 1911 that Rutherford conducted his gold foil experiment that demonstrated the presence of the nucleus in the atom. Just two years later, in 1913, English physicist Henry Moseley (1887-1915) examined x-ray spectra of a number of chemical elements. He would shoot X-rays through crystals of the element and study the wavelengths of the radiation he detected. Moseley found that there was a relationship between wavelength and atomic number. His results led to the definition of atomic number as the number of protons contained in the nucleus of each atom. He then realized that the elements of the periodic table should be arranged in order of increasing atomic number rather than increasing atomic mass.

When ordered by atomic number, the discrepancies within Mendeleev’s table disappeared. Tellurium has an atomic number of 52, while iodine has an atomic number of 53. So even though tellurium does indeed have a greater atomic mass than iodine, it is properly placed before iodine in the periodic table. Mendeleev and Moseley are credited with being most responsible for the modern periodic law : When elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties. The result is the periodic table as we know it today. Each new horizontal row of the periodic table corresponds to the beginning of a new period because a new principal energy level is being filled with electrons. Elements with similar chemical properties appear at regular intervals, within the vertical columns called groups .
Summary
- Elements of the periodic table are arranged in order of increasing atomic number.
- The periodic law states “When elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties.”
Practice
Use the link below to answer the following questions:
http://www.famousscientists.org/henry-moseley/
- Where did Moseley attend college?
- Who did he do research with after graduating from college?
- What is Moseley’s law?
Review
- Did Mendeleev know about the nucleus of an atom?
- Who discovered the relationship between wavelength of X-rays and atomic number?
- What did Moseley conclude from his research?
- What is the “periodic law”?
- What do the vertical columns (groups) in the periodic table represent?
Glossary
- group: Elements with similar chemical properties appear at regular intervals, within the vertical columns.
- period: A period is a horizontal row of the periodic table.
- periodic law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties.
Modern Periodic Table: Periods and Groups
Learning Objectives
Describe the organization of the modern periodic table.
How has the English dictionary evolved over time?
Language changes with time. New words enter the language and old words often disappear from lack of use. Dictionaries are published so that people can keep up with changes in language and know how to use words properly. These publications may be in print, as is the law dictionary below, or they may be electronic. Dictionaries can be found on the internet and apps are available for smartphones. Dictionaries are invaluable for good, reliable communication.
The Modern Periodic Table
The periodic table has undergone extensive changes in the time since it was originally developed by Mendeleev and Moseley. Many new elements have been discovered, while others have been artificially synthesized. Each fits properly into a group of elements with similar properties. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical column or group.
The figure below shows the most commonly used form of the periodic table. Each square shows the chemical symbol of the element along with its name. Notice that several of the symbols seem to be unrelated to the name of the element: Fe for iron, Pb for lead, etc. Most of these are the elements that have been known since ancient times and have symbols based on their Latin names. The atomic number of each element is written above the symbol.

A period is a horizontal row of the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. A new period begins when a new principal energy level begins filling with electrons. Period 1 has only two elements (hydrogen and helium), while periods 2 and 3 have 8 elements. Periods 4 and 5 have 18 elements. Periods 6 and 7 have 32 elements because the two bottom rows that are separated from the rest of the table belong to those periods. They are pulled out in order to make the table itself fit more easily onto a single page.
A group is a vertical column of the periodic table, based on the organization of the outer shell electrons. There are a total of 18 groups. There are two different numbering systems that are commonly used to designate groups and you should be familiar with both. The traditional system used in the United States involves the use of the letters A and B. The first two groups are 1A and 2A, while the last six groups are 3A through 8A. The middle groups use B in their titles. Unfortunately, there was a slightly different system in place in Europe. To eliminate confusion the International Union of Pure and Applied Chemistry (IUPAC) decided that the official system for numbering groups would be a simple 1 through 18 from left to right. Many periodic tables show both systems simultaneously.
Summary
- The periodic table is arranged in order of atomic number
- A period is a horizontal row of the periodic table.
- A group is a vertical row of the periodic table.
Practice
Use the link below to answer the following questions:
http://pontotriplo.org/triplepoint/2007/05/the_best_55_online_periodic_tables.html
- Select the periodic table from the “best” column that you like the most. Why did you choose that table?
- Which periodic table in the “specific” section do you like the most? Why?
- Which of the “funny” periodic tables do you like most? Why?
Review
- How is today’s periodic table different from the one that Mendeleev published?
- Are all the elements in today’s periodic table naturally occurring? Explain your answer.
- What is a “period?” What does it represent?
- What is a “group?” What does it represent?
- Why are there two different numbering systems for groups?
Glossary
- group: Elements with similar chemical properties appear at regular intervals, within the vertical columns.
- period: A period is a horizontal row of the periodic table.
Metals
Learning Objectives
- List properties of metals.
- List common metals and their uses.
Can you guess what types of metals these screws are made of?

Screws come in all sizes and shapes. They are all (well, almost all) made of some kind of metal. But they have differences in size, shape, and type of metal. Physical characteristics also differ. Some screws are long, and others are short. One screw may have a flat-head slot while another screw may have a Phillips-head. Some of the screws in the picture below are used to fasten things together, and others are used to hang heavy objects on a wall.
Chemists classify materials in many ways. We can sort elements on the basis of their electron arrangements. The way the electrons are distributed determines the chemical properties of the element. Another way is to classify elements based on physical properties. Some common physical properties are color, volume, and density. Other properties that allow us to sort on the basis of behavior are conduction of heat and electricity, malleability (the ability to be hammered into very thin sheets), ductility (the ability to be pulled into then wires), melting point, and boiling point. Three broad classes of elements based on physical properties are metals, nonmetals, and metalloids.
A metal is an element that is a good conductor of heat and electricity. Metals are also malleable, which means that they can be hammered into very thin sheets without breaking. They are ductile, which means that they can be drawn into wires. When a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. This is called luster. All metals are solid at room temperature with the exception of mercury (Hg), which is a liquid. Melting points of metals display a very wide variance. The melting point of mercury is -39°C, while the highest melting metal is tungsten (W), with a melting point of 3422°C. The elements in blue in the periodic table below are metals. About 80 percent of the elements are metals.

Gold has been used by many civilizations for making jewelry (see Figure 3). This metal is soft and easily shaped into a variety of items. Since gold is very valuable and often used as currency, gold jewelry has also often represented wealth.
Copper is a good conductor of electricity and is very flexible and ductile. This metal is widely used to conduct electric current in a variety of appliances, from lamps to stereo systems to complex electronic devices (see Figure 4).
Mercury is the only metal to exist as a liquid at room temperature (see Figure 5). This metal was extensively used in thermometers for decades until information about its toxicity became known. Mercury switches were once common, but are no longer used. However, new federally-mandated energy-efficient light bulbs that are now used contain trace amounts of mercury and represent a hazardous waste.

Figure 3. Gold jewelry. |

Figure 4. Copper wire exposed. |

Figure 5. Pouring Mercury. |
Summary
- Metals are good conductors of heat and electricity.
- Metals are malleable and ductile
- All metals are solids at room temperature with the exception of mercury
- Gold, silver, iron, and mercury are typical metals.
Practice
Select two metals from the periodic table that have not been mentioned. Locate information to answer the following questions:
- What color is this metal?
- Describe its ductility and malleability
- List three current uses for this metal.
Review
- What properties of an element are affected by electron distribution?
- Define malleability.
- Define ductility.
- State one reason gold is used in jewelry.
- Why is mercury no longer used in many devices?
Glossary
- conductor: A material that allows the flow of an electric current.
- ductile: A material that can be drawn into wires.
- malleable: Means that the material can be hammered into very thin sheets without breaking.
- metal: An element that is a good conductor of heat and electricity.
Nonmetals
Learning Objectives
- Define non-metal.
- List typical non-metals and their uses.

When we sort parts in our shop or garage, we often classify them in terms of common properties. One container might hold all the screws (possibly sub-divided by size and type). Another container would be for nails. Maybe there is a set of drawers for plumbing parts.
When you get finished, you could also have a collection of things that don’t nicely fit a category. You define them in terms of what they are not. They are not electrical components, or sprinkler heads for the yard, or parts for the car. These parts may have some common properties, but are a variety of items.
In the chemical world, these “spare parts” would be considered non-metals, loosely defined as not having the properties of metals. A nonmetal is an element that is generally a poor conductor of heat and electricity. Most properties of nonmetals are the opposite of metals. There is a wider variation in properties among the nonmetals than among the metals. Nonmetals exist in all three states of matter. The majority are gases, such as nitrogen and oxygen. Bromine is a liquid. A few are solids, such as carbon and sulfur. In the solid state, nonmetals are brittle , meaning that they will shatter if struck with a hammer. The solids are not lustrous. Melting points are generally much lower than those of metals. The green elements in the table below are non-metals.

Non-metals have a wide variety of uses. Sulfur can be employed in gunpowder, fireworks, and matches to facilitate ignition (see Figure 6). This element is also widely used as an insecticide, a fumigant, or a means of eliminating certain types of fungus. An important role for sulfur is the manufacture of rubber for tires and other materials. First discovered in 1839 by Charles Goodyear, the process of vulcanization makes the rubber more flexible and elastic as well as being more resistant to changes in temperature. A major use of sulfur is for the preparation of sulfur-containing compounds such as sulfuric acid.

Figure 6. Sulfur.
Bromine is a versatile compound, used mainly in manufacture of flame-retardant materials, especially important for children’s clothing (see Figure 7).

Figure 7. Bromine.
For treatment of water in swimming pools and hot tubs, bromine is beginning to replace chlorine as a disinfectant because of its higher effectiveness. When incorporated into compounds, bromine atoms play important roles in pharmaceuticals for treatment of pain, cancer, and Alzheimer’s disease.
Helium is one of the many non-metals that is a gas. Other non-metal gases include hydrogen, fluorine, chlorine, and all the group eighteen noble (or inert) gases. Helium is chemically non-reactive, so it is useful for applications such as balloons (see Figure 8) and lasers, where non-flammability is extremely important. Liquid helium exists at an extremely low temperature and can be used to cool superconducting magnets for imaging studies (MRI, magnetic resonance imaging). Leaks in vessels and many types of high-vacuum apparatus can be detected using helium. Inhaling helium changes the speed of sound, producing a higher pitch in your voice. This is definitely an unsafe practice and can lead to physical harm and death.

Figure 8. Blimp.
Summary
- Non-metals are generally poor conductors of heat and electricity.
- Properties of non-metals are usually the opposite of properties of metals
- Non-metals can be solid, liquid, or gas at room temperature depending upon the element.
- Sulfur, bromine, and helium are typical non-metals.
Practice
Select two non-metals from the periodic table that have not been mentioned. Locate information to answer the following questions:
- What are the physical properties of this non-metal?
- List three current uses for this element.
Review
- What are the properties of non-metals?
- List the states of matter in which non-metals can exist and give one example of each state
- What are the physical properties and uses of sulfur?
- What are the physical properties and uses of bromine?
- What are the physical properties and uses of helium?
Glossary
- non-metal: An element that is generally a poor conductor of heat and electricity.
- brittle: Shatters easily.
Metalloids
Learning Objectives
- Define metalloid.
- List common metalloids and give their uses.
Have you ever taken a multiple-choice test?

Most of the time the answers are specific choices – is the answer possibility a or possibility b? Quite often you can “think through” the choices to come up with the correct answer. More frustrating is the choice “none of the above.” You feel very uncertain checking that possibility.
Some elements are “none of the above.” They don’t fit neatly into the categories of metal or non-metal because of their characteristics. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids can also be called semimetals. On the periodic table, the elements colored yellow, which generally border the stair-step line, are considered to be metalloids. Notice that aluminum borders the line, but it is considered to be a metal since all of its properties are like those of metals.

Examples of Metalloids

Figure 9. Silicon.
Silicon is a typical metalloid (see Figure 9). It has luster like a metal, but is brittle like a nonmetal. Silicon is used extensively in computer chips and other electronics because its electrical conductivity is in between that of a metal and a nonmetal.
Boron is a versatile element that can be incorporated into a number of compounds (see Figure 10). Borosilicate glass is extremely resistance to thermal shock. Extreme changes in the temperature of objects containing borosilicates will not create any damage to the material, unlike other glass compositions, which would crack or shatter. Because of their strength, boron filaments are used as light, high-strength materials for airplanes, golf clubs, and fishing rods. Sodium tetraborate is widely used in fiberglass as insulation and also is employed in many detergents and cleaners.
Arsenic has long played a role in murder mysteries, being used to commit the foul deed (see Figure 11). This use of the material is not very smart since arsenic can be easily detected on autopsy. We find arsenic in pesticides, herbicides, and insecticides, but the use of arsenic for these applications is decreasing due to the toxicity of the metal. Its effectiveness as an insecticide has led arsenic to be used as a wood preservative.
Antimony is a brittle, bluish-white metallic material that is a poor conductor of electricity (see Figure 12). Used with lead, antimony increases the hardness and strength of the mixture. This material plays an important role in the fabrication of electronic and semiconductor devices. About half of the antimony used industrially is employed in the production of batteries, bullets, and alloys.

Figure 10. Boron. |

Figure 11. Arsenic. |

Figure 12. Antimony. |
Summary
- Metalloids are elements with properties intermediate between those of metals and non-metals
- Silicon is a metalloid because it has luster, but is brittle.
- Boron, arsenic, and antimony are metalloids with a variety of uses.
Practice
Use the link below to answer the following:
http://sciencestruck.com/uses-of-metalloids
- Describe the physical properties of two metalloids of your choosing.
- List two uses for each of these metalloids.
Review
- Define “metalloid.”
- Why would it be difficult to decide whether or not an element was a metalloid?
- Why is silicon used extensively in electronics?
- What are borosilicates used for?
- Why is the use of arsenic as an insecticide decreasing?
- What is a main application of antimony?
Glossary
- metalloid: An element that has properties that are intermediate between those of metals and nonmetals.
- luster: A reflective surface.
Blocks of the Periodic Table
Learning Objectives
- Identify blocks of the periodic table.
- Determine the block each element belongs in by its electron configuration.
What makes these music notes unique?

We all enjoy music of some sort. Some people like classical music, others like jazz or country. Music styles change from one period of time to the next, and from one region to another. Each type of music has its language that describes it. Classical music has a certain structure, style, and content. There are different expressions of classical music – the symphony, concerto, sonata. We have ballet and opera as well as choral music. Jazz has a different set of characteristics from classical and different styles of performance. Each type of music can be described and compared to other types on the basis of certain common qualities like notes, chords, and melodic styles.
The elements in the periodic table could be considered to be similar to types of music. Each set of elements has its unique set of properties, with different sets of elements having some common characteristics in terms of electron arrangements. We can see patterns of electronic structure and reactivity in the periodic table that allow us to understand better the behavior of individual elements.
Periods and Blocks
There are seven horizontal rows of the periodic table, called periods. The length of each period is determined by the number of electrons that are capable of occupying the sublevels that fill during that period, as seen in the Table below .
| Period | Number of elements in period | Sublevels in order of fill |
| 1 | 2 | 1 s |
| 2 | 8 | 2 s 2 p |
| 3 | 8 | 3 s 3 p |
| 4 | 18 | 4 s 3 d 4 p |
| 5 | 18 | 5 s 4 d 5 p |
| 6 | 32 | 6 s 4 f 5 d 6 p |
| 7 | 32 | 7 s 5 f 6 d 7 p |
Recall that the four different sublevels each consist of a different number of orbitals. The s sublevel has one orbital, the p sublevel has three orbitals, the d sublevel has five orbitals, and the f sublevel has seven orbitals. In the first period, only the 1 s sublevel is being filled. Since all orbitals can hold two electrons, the entire first period consists of just two elements. In the second period, the 2 s sublevel, with two electrons, and the 2 p sublevel with six electrons, are being filled. Consequently, the second period contains eight elements. The third period is similar to the second, filling the 3 s and 3 p sublevels. Notice that the 3 d sublevel does not actually fill until after the 4 s sublevel. This results in the fourth period containing 18 elements due to the additional 10 electrons that are contributed by the d sublevel. The fifth period is similar to the fourth. After the 6 s sublevel fills, the 4 f sublevel with its 14 electrons fills. This is followed by the 5 d and the 6 p . The total number of elements in the sixth period is 32. The later elements in the seventh period are still being created. So while there are a possible of 32 elements in the period, the current number is slightly less.
The period to which a given element belongs can easily be determined from its electron configuration. For example, consider the element nickel (Ni). Its electron configuration is [Ar]3 d 8 4 s 2 . The highest occupied principal energy level is the fourth, indicated by the 4 in the 4 s 2 portion of the configuration. Therefore, nickel can be found in the fourth period of the periodic table.
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The s , p , d , and f blocks are illustrated below.

The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs. In other words, the 3 d sublevel fills during the fourth period. The f sublevel is always two levels behind. The 4 f sublevel belongs to the sixth period.
Summary
- The horizontal rows of the periodic table are called periods.
- The length of a period depends on how many electrons are needed to occupy the sublevels that fill the period.
- Blocks indicate which sublevel is being filled.
Practice
Use the link below to answer the following questions:
http://en.wikibooks.org/wiki/High_School_Chemistry/The_Periodic_Table_and_Electron_Configurations
- How many elements are in the second period? The fourth? The sixth?
- Use a periodic table to identify the block that each of these elements would be found.
- rubidium
- holmium
- palladium
- tellurium
Review
- What are the horizontal rows of the periodic table called?
- Which sublevel is being filled in period 1?
- Which sublevel is being filled in period 7?
- How does the electron configuration of an element give information about the period it is in?
- What block of elements has the d sublevels being filled?
Glossary
- block: The periodic table can be divided into blocks denoting which sublevel is in the process of being filled.
- period: Each horizontal row of the seven rows of the periodic table.
- sublevel: Electron orbitals s, p, d or f.
Hydrogen and Alkali Metals
Learning Objectives
- Indicate the group in which the alkali metals are located.
- List the alkali metals.
- Describe the valence shell electron configuration for the hydrogen atom and the alkali metal elements.
Can you guess what kind of reaction is taking place in this picture?

Some chemistry students just enjoy learning about the science, while others are intrigued by the violent reactions that sometimes can occur. Many chemistry classes have been enlivened by the demonstration of how reactive sodium is with water. In some instances, the demonstration has gone off safely. Unfortunately, in other situations students and instructors have incurred serious injury due to their failure to observe proper safety precautions.
One value of the periodic table is the ability to make predictions about the behavior of individual elements. By knowing which group an element is in, we can determine the number of reactive electrons and say something about how that element will behave.
The periodic table is arranged on the basis of atomic numbers (number of protons in the nucleus). One of the valuable consequences of this arrangement is that we can learn a lot about the electron distribution in these atoms. The colors in the table below indicate the different groupings of atoms based on the location and number of electrons in the atom.
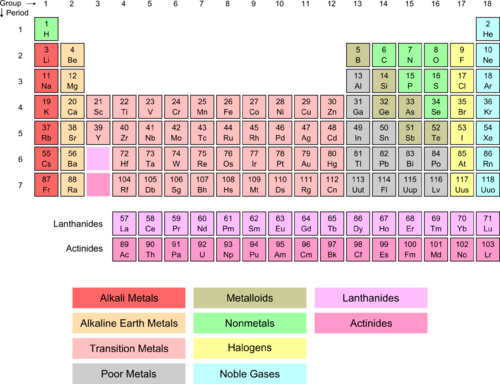
If we look at Group I (red column), we see that it is labeled alkali metals . Also note the green H above the alkali metals. All of these elements have a similar configuration of outer-shell electrons (see Table below ). In each case, there is one electron in the outer orbital and that is an s -orbital electron. Hydrogen is not an alkali metal itself, but has some similar properties due to its simple one proton (loctated in the nucleus), one electron arrangement. The lone electron exists in a s -orbital around the nucleus. For lithium, there are two 1 s electrons in an inner orbit and one 2 s electron in the outer orbit. The same pattern holds for sodium and potassium.
| Element | Symbol | Electron Configuration |
| hydrogen | H | 1 s 1 |
| lithium | Li | [He]2 s 1 |
| sodium | Na | [Ne]3 s 1 |
| potassium | K | [Ar]4 s 1 |
| rubidium | Rb | [Kr]5 s 1 |
| cesium | Cs | [Xe]6 s 1 |
| francium | Fr | [Rn]7 s 1 |

Figure 13. Cesium Orbitals.
Even an atom with a very complex electron composition such as cesium still has the single s electron in its outer orbital (see Figure 13).
This one electron is very easily removed during chemical reactions. The group I elements react rapidly with oxygen to produce metal oxides. They are very soft metals, which become liquid just above room temperature.
The alkali metals also react readily with water to produce hydrogen gas and metal hydroxides in the following video: Alkali Metals: Explosive reactions.
Li reacts with water to produce hydrogen gas. Sodium also reacts the same way, just more rapidly. Potassium reacts rapidly with water producing hydrogen gas and heat which ignites the hydrogen gas. Rubidium and cesium react yet more vigorously and explode on contact with water.
Summary
- Group I (alkali metals and H) elements all have one electron in their outer shell.
- This electron is in a s orbital.
- The Group I metals are all very reactive with water.
Practice
Read this page on Group 1: Hydrogen and the Alkali Metals to answer the following questions:
- How is hydrogen similar to the alkali metals? How is it different?
- Why don’t we know much about francium (atomic number 87)?
- Describe the physical properties of the alkali metals.
Review
- What group are the alkali metals and hydrogen in?
- What is the outer shell electron configuration in this group?
- Which alkali metal is a liquid at room temperature?
- How reactive are the alkali metals with oxygen?
- How reactive are these metals with water?
Groups
- alkali metal: Has one electron in their outer shell and can be found in Group I in the periodic table.
Alkaline Earth Metals
Learning Objectives
- List the alkaline earth elements.
- Give the electron configuration of this group.
- Describe reaction of the alkaline earth elements.
How are oyster shells and chemistry related?

We take a lot of chemistry for granted. Very few of us think about the chemistry of bone or oyster shells. Both of these materials have large amounts of calcium compounds in them and play important roles in maintaining the structure of the organism. The shell provides a solid surrounding for the oyster. Bones give support to the body so the person can move around and not just be a soft mass of tissue.
Group 2 elements are referred to as “alkaline earth” metals (tan column below). The name “alkaline” comes from the fact that compounds of these elements form basic (pH greater than 7) or alkaline solutions when dissolved in water. If the Group 1 elements all have one s electron in their outer orbital, we can predict that the Group 2 elements will have two electrons in that outer shell.

The beryllium atom, the first element of Group 2, has an atomic number of four. The atom has the 1 s shell filled as well as the 2 s shell, giving a total of four electrons (1 s 2 2 s 2 ). Note that there are two s electrons in the outer shell, a structure that is characteristic of the Group 2 elements. Barium (atomic number 56) has the same outer shell structure of two electrons in the s orbital, even though the internal electron structure for barium is quite complicated.

Radium (atomic number 88) has similar properties to barium and is also in the Group 2 category. However, radium is a radioactive element and is generally under the category of radioisotopes in addition to being an alkaline earth metal, because it is not a stable element.
The Group 2 elements tend to be less reactive than their Group 1 counterparts. The need to remove two electrons in order for the material to react means more energy is needed for electron removal. However, these elements are reactive enough that they do not exist in their elemental forms in nature, but are present as compounds.
Uses of Alkaline Earth Compounds
Since magnesium burns brightly, it is used in flares and fireworks. Magnesium alloys with aluminum provide light weight and sturdy materials for airplanes, missiles, and rockets. Several antacids use magnesium hydroxide to neutralize excess stomach acid.
Calcium compounds are widely found in limestone, marble, and chalk. Calcium is an important constituent of cement. Other uses include calcium chloride as a deicer and limestone as a white pigment in paints and toothpaste.
Strontium is widely used in fireworks and magnets. Barium compounds can be used in paints, filler for rubber, plastic, and resins, and as a contrast medium for X-rays. Many beryllium compounds are toxic, but these materials have been employed in metal alloys.
Summary
- The alkaline earth elements are in Group 2 of the periodic table.
- These elements each have two s electrons in their outer shell.
- The alkaline earth elements are less reactive than the alkali metals.
Practice
Browse the site below to answer the following questions:
http://www.rsc.org/periodic-table
- What color are all the alkaline earth elements?
- In what compounds is magnesium found in nature?
- In what compounds is calcium found in nature?
Review
- Why are these elements known as “alkaline earth” elements?
- How many electrons are in the outer shell of the alkaline earth elements?
- Are the alkaline earth elements more or less reactive than the alkali metals? Explain your answer?
- Is radium usually considered as part of the alkaline earth category in terms of chemistry? Explain your answer.
Glossary
- alkaline: Comes from the fact that compounds of these elements from basic or alkaline solutions when dissolved in water.
- alkaline earth: Group II elements in the periodic table.
- basic: pH greater than 7.
Noble Gases
Learning Objectives
- List the noble gases.
- Describe the electron configuration of the noble gases.
- Describe compounds of the noble gases.
What gives these lights their color?

Cities at night would be rather boring without all the bright lights. They provide colorful Illuminations and help make things much more visible. We call these lights “neon lights,” but they use several gases to make the different colors.
The reactivity of an element can give us important clues as to the electron configuration of that material. If an element is extremely unreactive, this suggests that the electron configuration is such that adding or removing electrons is very unlikely. There must be a stable electron configuration that resists further reaction.

The Group VIII (new group 18) elements are essentially chemically inert (light blue column on the right). All these elements exist as monatomic gases at room temperature. If we look at the electron configurations, we see that helium (atomic number 2) has a full shell of two s electrons. Since there are no electrons shielding this shell from the nucleus, these two electrons will be very difficult to remove, making helium unreactive.
The remaining elements in the group have full outer shells consisting of two s electrons and six p electrons for an outer shell content of eight electrons. This particular arrangement renders the atoms fairly unreactive. This group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Another popular term is “ noble gases ,” suggesting that these gases do not like to have much to do with the other, more common materials (or that they don’t do a lot of work).
Noble Gas Compounds
In more recent years, a number of reactions using the noble gas elements have been discovered. Although the conventional wisdom was that the complete outer shells of these elements would not allow them to react, some scientists believed that the outer electrons of the larger elements (such as Rn, Xe, and Kr) were far enough away from the nucleus that they should be able to be displaced under the right set of conditions. The first compound (XePtF6 ) was made with xenon in 1962. Since then, several compounds have been made with radon, xenon, krypton, and argon. Only helium and neon have not formed compounds at this time.
Colors of Noble Gases
The different gases glow when an electric current is passed through them. Many of these gases are used in displays because of their chemical inertness. They are stable and will not react with other materials in the system. Radon also will give a reddish glow, but is not used because it is radioactive and will not retain its structure as radon for any significant length of time.

Summary
- The noble gases are in Group VIII of the periodic table.
- Helium has a full outer shell of two
 electrons.
electrons. - The other gases have full outer shells of two s and six p electrons.
- Compounds have been formed with Rn, Xe, Kr, and Ar.
Practice
Use the link below to answer the following questions:
http://www.learner.org/interactives/periodic/groups4.html
- Where was helium discovered? What does the name mean?
- What does krypton react with?
- What does xenon react with?
- List present-day uses for each of the noble gases.
Review
- What elements comprise the noble elements?
- What state are they in at room temperature?
- Why is helium non-reactive?
- Why were the other noble gases believed to be non-reactive?
- When was the first compound formed from xenon?
- What happens when an electric current is passed through these gases?
Glossary
- inert: Chemically unreactive and can be found in Group VIII in the period table.
- monatomic: Has one atom.
- noble gas: Another term for inert gas.
Halogens
Learning Objectives
- Name the halogens.
- Describe the physical properties of the halogens.
- Describe the chemical properties of the halogens.
How do you study a gas that does not exist as such in nature? It’s not as easy as you think. Fluorine is so reactive that we cannot find it free in nature. None of the halogens exist free in nature (unlike some of the metals suchas gold and silver) because they are very reactive. The video below shows how violently elemental fluorine reacts with other materials.
Some elements are much more reactive than others. The Group I (red) and Group II (tan) elements can easily lose electrons during a reaction. Elements of other groups are much more likely to accept electrons as they react.

The elements of Group VIIA (new Group 17 – fluorine, chlorine, bromine, iodine, and astatine) are called the halogens (tan column). The term “halogen” means “salt-former” because these elements will readily react with alkali metal and alkaline earth metals to form halide salts. The halogens all have the general electron configuration ns 2 np 5 , giving them seven valence electrons. They are one electron short of having the full outer s and p sublevel, which makes them very reactive.
Physical Properties of Halogens

As elements, chlorine and fluorine are gases at room temperature, bromine is a dark orange liquid, and iodine is a dark purple-gray solid. Astatine is so rare that its properties are mostly unknown. In the picture below we see chlorine gas on the left (green), bromine solid and vapor in the middle (orange), and solid iodine (grey) on the right. Fluorine is not shown in the picture below because it is too corrosive and will destroy the glass container.

None of these elements are found free in nature because of their reactivity. Fluorine is found in combination with cations in several minerals. Chlorine is found in table salt, in the oceans (which are about 2% chloride ion by weight) and in lakes such as the Great Salt Lake in Utah. Small amounts of bromide and iodide salts can be found in the oceans and in brine wells in several states.
Watch the following two video experiments of p block elements:
This first video is of bromine reacting with aluminum:
This second video shows halogen reactions:
http://youtu.be/cbFCWFksYoM
Summary
- The halogens all have seven electrons in their outer shells.
- The electron configuration in the outer shell is ns 2 np 5 .
- As the atomic number increases, the reactivity of the halogens decreases.
- Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid.
Practice
Use the link below to answer the following questions:
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/group7.php
- Are the halogen elements monatomic or diatomic molecules?
- Why do we not know very much about astatine?
- Describe the reactivity of elemental fluorine.
- List one use for fluorine, chlorine, bromine, and iodine compounds.
Review
- List the halogens by name, chemical symbol, and atomic weight.
- What does the term “halogen” mean?
- What is the outer shell electron configuration for the halogens?
- What is the physical state of each halogen at room temperature?
- Where are the halogens found?
Glossary
- halogen: The term “halogen” means “salt-former” because these elements will readily react with alkali metal and alkaline earth metals to form halide salts. These elements can be found in Group VII in the periodic table.
Transition Elements
Learning Objectives
- Identify the transition metals on the periodic table.
- Describe the characteristic electron configuration of the transition elements.
What are the similarities and differences between these two cars?

From the outside, the two cars above look the same (except for the flashy paint job on the racing model). They are the same model of the car, but one is a stock edition for regular driving while the other one is built for high-speed racing. We really can’t tell much from the external view. To see the differences, we need to go under the hood, take the engines apart, and look at the braking and suspension systems in order to see how the two cars differ.
Many electron configurations of elements are simple and straightforward. We can look at the outer shell and easily understand how that set of elements will react in terms of electron gain or loss. However, there are sets of elements that are more complex in their behavior. One such group is called the transition elements.

Transition elements are the elements that are found in Groups 3-12 (old groups IIA-IIB) on the periodic table (salmon-colored block in the middle of the table). The term refers to the fact that the d sublevel , which is in the process of being filled, is in a lower principal energy level than the ![]() sublevel filled before it. For example, the electron configuration of scandium, the first transition element, is [Ar]3d14s2.
sublevel filled before it. For example, the electron configuration of scandium, the first transition element, is [Ar]3d14s2.

Figure 14. Piece of silver.
Remember that the configuration is reversed from the fill order—the 4 s filled before the 3 d begins. Because they are all metals, the transition elements are often called the transition metals. As a group, they display typical metallic properties and are less reactive than the metals in Groups 1 and 2. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined state. These include platinum, gold, and silver. Because of this unique filling order, the transition elements are often referred to as “ d -block” elements.
Compounds of many transition elements are distinctive for being widely and vividly colored. As visible light passes through a transition metal compound dissolved in water, the d-orbitals absorb light of various energies. The visible light of a given energy level which is not absorbed produces a distinctly colored solution.

Figure 15. Transition metal compounds dissolved in water exhibit a wide variety of bright colors. From left to right are shown solutions of cobalt(II) nitrate, potassium dichromate, potassium chromate, nickel(II) chloride, copper(II) sulfate, and potassium permanganate.
Summary
- The transition elements are found in groups IIIA-IIB (new groups 3-12).
- These elements are characterized by having unfilled d sublevels.
- In general, the next higher s sublevel is already filled or has one electron missing.
- Many transition element compounds are brightly colored due to the inner-level d electron transitions.
Practice
Use the link below to answer the following questions:
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch12/trans.php
- Why are the transition metals named “transition?”
- How do the properties of transition metals and main-group metals overlap?
- Which electrons are more likely to be removed from transition metals as they react?
Review
- List five different transition elements, giving their name, chemical symbol, and atomic number.
- What is unique about the transition elements in terms of electron configurations?
- Why are these elements often referred to as “ d -block” elements?
- Which transition group elements can be found in their free state in nature?
- Why do many transition element compounds have bright colors?
Glossary
- transition element: Elements can be found in Groups 3-12 (old groups IIA-IIB) on the period table. The term refers to the fact that the d sublevel, which is in the process of being filled, is in a lower principal energy level than the s sublevel filled before it.
- sublevel: Electron orbitals known as s, p, d, or f.
Lanthanides and Actinides
Learning Objectives
- Describe the electron configurations of the lanthanide and actinide elements.
- List uses for lanthanides and actinides.
How many dolls are in this picture?

Russian “nesting dolls” (often known as matryoshka dolls) have a long history in Russia. These dolls are designed to nest inside of one another. When we open the largest doll, we find a somewhat smaller doll inside it. These dolls can often go down seven or eight layers. The set seen above is unusual in that it has over thirty-five layers.
We see some hidden “layers” in chemistry. As we look at the periodic table below, we see two pink boxes – one between Ba (element 56) and Hf (element 72) and the other between Ra (88) and Rf (104). These elements all have unfilled f -sublevels. Because of the uniqueness of the electron configurations, these elements fit into the two boxes in the larger periodic table.
As the number of elecrons in an atom increases, we begin to see some strange behaviors. Due to the way the electron energy levels work, some inner levels fill after one or more outer levels do. We see this in two similar groups of elements – the lanthanides and the actinides .

The f-Block
The first of the f sublevels to begin filling is the 4 f sublevel. It fills after the 6 s sublevel, meaning that f sublevels are two principal energy levels behind. The general electron configuration for elements in the f block is ( n – 2 )f 1-14 ns 2 . The seven orbitals of the f sublevel accommodate 14 electrons, so the f block is 14 elements in length. It is pulled out of the main body of the period table and is shown at the very bottom. Because of that, the elements of the f block do not belong to a group, being wedged in between Groups 3 and 4. The lanthanides are the 14 elements from cerium (atomic number 58) to lutetium (atomic number 71). The word comes from the Greek “lanthanein” meaning “to be hidden.” The name probably arose because these elements all hide behind one another in the periodic table. The 4 f sublevel is in the process of being filled for the lanthanides. They are all metals and are similar in reactivity to the Group 2 alkaline earth metals.
The actinides are the 14 elements from thorium (atomic number 90) to lawrencium (atomic number 103). The 5 f sublevel is in the process of being filled. The actinides are all radioactive elements and only the first four have been found naturally on Earth. All of the others have only been artificially made in the laboratory. The lanthanides and actinides together are sometimes called the inner transition elements.
Uses of Lanthanides
Lanthanides have been widely used as alloys to impart strength and hardness to metals. The main lanthanide used for this purpose is cerium, mixed with small amounts of lanthanum, neodymium, and praseodymium. These metals are also widely used in the petroleum industry for refining of crude oil into gasoline products.
Erbium and other lanthanides are widely used in some optical devices, such as night vision goggles, laser beams, and phosphorescent materials.

Figure 16. Oil refinery. |

Figure 17. Night vision goggles. |
Uses of Actinides
The actinides are valuable primarily because they are radioactive. These elements can be used as energy sources for applications as varied as cardiac pacemakers and generation of electrical energy for instruments on the moon. Uranium and plutonium have been employed in nuclear weapons and in nuclear power plants.

Figure 18. Pacemaker.
Summary
- Lanthanides and actinides are elements with unfilled f orbitals.
- Lanthanides are all metals with reactivity similar to group 2 elements.
- Actinides are all radioactive elements.
- Lanthanides are used in optical devices (night vision goggles), petroleum refining, and alloys.
- Actinides are found primarily in applications where their radioactivity can be used to power devices such as cardiac pacemakers.
Practice
Use the link below to answer the following questions:
https://www.thoughtco.com/lanthanides-properties-606651
- List three physical characteristics of the lanthanides
- What do lanthanides produce when they react with water?
Review
- What electron sublevel is being filled in the lanthanides?
- What electron sublevel is being filled in the actinides?
- What sublevel is filled just prior to the filling of this sublevel?
- Which actinides are found naturally on earth?
- List some uses for lanthanides.
- List some uses for actinides.
Glossary
- lanthanide: The lanthanides are the 14 elements from cerium (atomic number 58) to lutetium (atomic number 71). The word comes from the Greek “lanthanein” meaning “to be hidden.”
- actinide: The actinides are the 14 elements from thorium (atomic number 90) to lawrencium (atomic number 103). The 5f sublevel is in the process of being filled. The actinides are all radioactive elements and only the first four have been found naturally on Earth.
- f-block: In the periodic table, any element that has atoms or ions who have valence electrons in f-orbitals.
Periodic Trends: Atomic Radius
Learning Objectives
- Define atomic radius.
- Describe how the atomic changes within a period.
- Describe how the atomic radius changes within a group.
How can all of these people fit in such a small space?
 Events draw large numbers of people to them. Even an outdoor event can fill up so that there is no room for more people. The crowd capacity depends on the amount of space in the venue, and the amount of space depends on the size of the objects filling it. We can get more people into a given space than we can elephants, because the elephants are larger than people. We can get more squirrels into that same space than we can people for the same reason. Knowing the sizes of objects we are dealing with can be important in deciding how much space is needed.
Events draw large numbers of people to them. Even an outdoor event can fill up so that there is no room for more people. The crowd capacity depends on the amount of space in the venue, and the amount of space depends on the size of the objects filling it. We can get more people into a given space than we can elephants, because the elephants are larger than people. We can get more squirrels into that same space than we can people for the same reason. Knowing the sizes of objects we are dealing with can be important in deciding how much space is needed.
The size of atoms is important when trying to explain the behavior of atoms or compounds. One of the ways we can express the size of atoms is with the atomic radius. This data helps us understand why some molecules fit together and why other molecules have parts that get too crowded under certain conditions.
The size of an atom is defined by the edge of its orbital. However, orbital boundaries are fuzzy and in fact are variable under different conditions. In order to standardize the measurement of atomic radii, the distance between the nuclei of two identical atoms bonded together is measured. The atomic radius is defined as one-half the distance between the nuclei of identical atoms that are bonded together.

Figure 19. The atomic radius (r) of an atom can be defined as one half the distance (d) between two nuclei in a diatomic molecule.
Atomic radii have been measured for elements. The units for atomic radii are picometers, equal to 10 -12 meters. As an example, the internuclear distance between the two hydrogen atoms in an H 2 molecule is measured to be 74 pm. Therefore, the atomic radius of a hydrogen atom is ![]() .
.
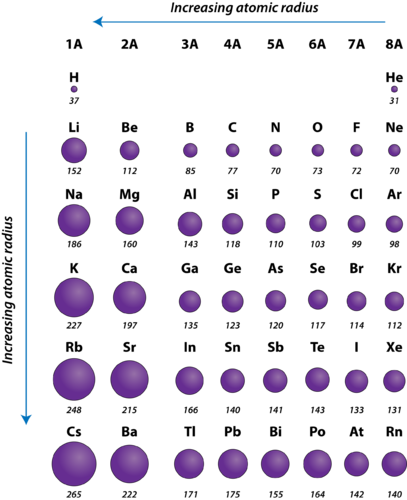
Figure 20. Atomic radii of the representative elements measured in picometers.
Periodic Trend
The atomic radius of atoms generally decreases from left to right across a period. There are some small exceptions, such as the oxygen radius being slightly greater than the nitrogen radius. Within a period, protons are added to the nucleus as electrons are being added to the same principal energy level. These electrons are gradually pulled closer to the nucleus because of its increased positive charge. Since the force of attraction between nuclei and electrons increases, the size of the atoms decreases. The effect lessens as one moves further to the right in a period because of electron-electron repulsions that would otherwise cause the atom’s size to increase.
Group Trend
The atomic radius of atoms generally increases from top to bottom within a group. As the atomic number increases down a group, there is again an increase in the positive nuclear charge. However, there is also an increase in the number of occupied principle energy levels. Higher principal energy levels consist of orbitals which are larger in size than the orbitals from lower energy levels. The effect of the greater number of principal energy levels outweighs the increase in nuclear charge and so atomic radius increases down a group.

Figure 21. A graph of atomic radius plotted versus atomic number. Each successive period is shown in a different color. As the atomic number increases within a period, the atomic radius decreases.
Summary
- Atomic radius is determined as the distance between the nuclei of two identical atoms bonded together.
- The atomic radius of atoms generally decreases from left to right across a period.
- The atomic radius of atoms generally increases from top to bottom within a group.
Practice
Use the link below to answer the following questions:
https://chem.libretexts.org/Reference/Periodic_Table_of_the_Elements/Atomic_Radi
- What influences the atomic size of an atom?
- What is a covalent radius?
- What is an ionic radius?
Review
- Define “atomic radius.”
- What are the units for measurement of atomic radius?
- How does the atomic radius change across a period?
- How does atomic radius change from top to bottom within a group?
- Explain why the atomic radius of hydrogen is so much smaller that the atomic radius for potassium.
Glossary
- atomic radius: The atomic radius is defined as one-half the distance between the nuclei of identical atoms that are bonded together.
Periodic Trends: Ionization Energy
Learning Objectives
- Define ionization energy.
- Describe factors affecting ionization energy.
- Describe how ionization energy changes across a period.
- Describe how ionization energy changes down a group.
Why do sheep travel in herds?

Like many other animals, sheep travel in herds. The tendency is for each individual sheep to stay with the herd. However, a sheep may sometimes wander off, depending on how strong the attraction is for a particular food or water supply. At other times, a sheep may become frightened and run off. Whether a sheep chooses to stay with the herd or go its own way depends on the balance between attraction to the herd and attraction to the outside influence.
There is an on-going tension between the electrons and protons in an atom. Reactivity of the atom depends in part on how easily the electrons can be removed from the atom. We can measure this quantity and use it to make predictions about the behaviors of atoms.
Ionization energy is the energy required to remove an electron from a specific atom. It is measured in kJ/mol, which is an energy unit, much like calories. The ionization energies associated with some elements are described in the Table below. For any given atom, the outermost valence electrons will have lower ionization energies than the inner-shell kernel electrons. As more electrons are added to a nucleus, the outer electrons become shielded from the nucleus by the inner shell electrons. This is called electron shielding.
| Element | IE 1 |
IE 2 | IE 3 | IE 4 | IE 5 | IE 6 |
| H | 1312 | |||||
| He | 2373 | 5251 | ||||
| Li | 520 | 7300 | 11,815 | |||
| Be | 899 | 1757 | 14,850 | 21,005 | ||
| B | 801 | 2430 | 3660 | 25,000 | 32,820 | |
| C | 1086 | 2350 | 4620 | 6220 | 38,000 | 47,261 |
| N | 1400 | 2860 | 4580 | 7500 | 9400 | 53,000 |
| O | 1314 | 3390 | 5300 | 7470 | 11,000 | 13,000 |
If we plot the first ionization energies vs. atomic number for the main group elements, we would have the following trend

Figure 22. Ionization energy and atomic number.
Moving from left to right across the periodic table, the ionization energy for an atom increases. We can explain this by considering the nuclear charge of the atom. The more protons in the nucleus, the stronger the attraction of the nucleus to electrons. This stronger attraction makes it more difficult to remove electrons.
Within a group, the ionization energy decreases as the size of the atom gets larger. On the graph, we see that the ionization energy increases as we go up the group to smaller atoms. In this situation, the first electron removed is farther from the nucleus as the atomic number (number of protons) increases. Being farther away from the positive attraction makes it easier for that electron to be pulled off.

Summary
- Ionization energy refers to the amount of energy needed to remove an electron from an atom.
- Ionization energy decreases as we go down a group.
- Ionization energy increases from left to right across the periodic table.
Practice
Read this page on Ionization Energy to answer the questions below:
- What is a “gaseous atom?”
- Why would the ionization energy for O be somewhat less than that for N?
- Why is a third-level electron easier to remove than a first-level one?
Review
- Define “ionization energy.”
- Do valence electrons have larger or smaller ionization energies that the inner-shell kernel electrons?
- What is electron shielding?
- Describe the trends in ionization energy from left to right across the periodic table.
- Describe the trends in ionization energy from top to bottom of a group in the periodic table.
- Why is the second ionization energy for lithium so much larger than the first ionization energy?
Glossary
- electron shielding: As more electrons are added to a nucleus, the outer electrons become shielded from the nucleus by the inner shell electrons
- ionization energy: The energy required to remove an electron from a specific atom. It is measured in kJ/mol, which is an energy unit, much like calories.
Electron Shielding
Learning Objectives
- Define electron shielding.
- Explain sublevel shielding.
What is the goal of a roller derby game?

Roller derby is a popular sport, although it is unfamiliar to many people. The basic purpose is to set one team member (the “jammer”) past the opposing team to score points. Other members of the team serve as blockers to prevent the opposing team from stopping the jammer. Blockers interfere with the interaction between the jammer and the opponents by getting between the jammer and the skaters trying to stop her.
The attraction between an electron and the nucleus of the atom is not a simple issue. Only with hydrogen is there a one-to-one relationship that can be discussed in terms of direct charge attraction. As the size of the atom increases, the number of protons and electrons also increase. These changes influence how the nucleus attracts electrons.
In general, the ionization energy of an atom will increase as we move from left to right across the periodic table. There are several exceptions to the general increase in ionization energy across a period. The elements of Group 13 (B, Al, etc.) have lower ionization energies than the elements of Group 2 (Be, Mg, etc.). This is an illustration of a concept called “electron shielding.” Outer electrons are partially shielded from the attractive force of the protons in the nucleus by inner electrons.

Figure 23. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. A larger shielding effect results in a decrease in ionization energy.
To explain how shielding works, consider a lithium atom. It has three protons and three electrons—two in the first principal energy level and its valence electron in the second. The valence electron is partially shielded from the attractive force of the nucleus by the two inner electrons. Removing that valence electron becomes easier because of the shielding effect.
There is also a shielding effect that occurs between sublevels within the same principal energy level. Specifically, an electron in the “s” sublevel is capable of shielding electrons in the “p” sublevel of the same principal energy level. This is because of the spherical shape of the “s” orbital. The reverse is not true – electrons in “p” orbitals do not shield electrons in “s” orbitals.

Figure 24. The spherical 3s orbital exhibits a shielding effect on the dumbbell shaped 3p orbital that is of slightly higher energy. This reduces the ionization energy of a 3p electron compared to a 3s electron.
The electron being removed from an Al atom is a 3p electron, which is shielded by the two 3s electrons as well as all the inner core electrons. The electron being removed from a Mg atom is a 3s electron, which is only shielded by the inner core electrons. Since there is a greater degree of electron shielding in the Al atom, it is slightly easier to remove the valence electron and its ionization energy is less than that of Mg. This is despite the fact that the nucleus of the Al atom contains one more proton than the nucleus of the Mg atom .
There is another anomaly between Groups 15 and 16. Atoms of Group 16 (O, S, etc.) have lower ionization energies than atoms of Group 15 (N, P, etc.). Hund’s rule is behind the explanation. In a nitrogen atom, there are three electrons in the 2p sublevel and each is unpaired. In an oxygen atom, there are four electrons in the 2p sublevel, so one orbital contains a pair of electrons. It is that second electron in the orbital that is removed in the ionization of an oxygen atom. Since electrons repel each other, it is slightly easier to remove the electron from the paired set in the oxygen atom than it is to remove an unpaired electron from the nitrogen atom.
Summary
- Electron shielding refers to the blocking of valence shell electron attraction by the nucleus due to the presence of inner-shell electrons.
- Electrons in an s orbital can shield p electrons at the same energy level because of the spherical shape of the s orbital.
- Electrons in paired spin configurations are slightly easier to remove than unpaired electrons.
Practice
Use the link below to answer the following questions:
http://www.wisegeek.org/what-is-the-shielding-effect.htm
- Why are electrons highly attracted to the nucleus?
- What happens when additional electrons are present in different orbits?
- What electrons are mainly influenced by electron shielding?
Review
- Define “electron shielding.”
- Why do group 13 elements have lower ionization energies than group 2 elements?
- What influence does a larger shielding effect have on ionization energy?
- How do s orbit electrons affect the ionization energy of a p electron in the same shell?
- Why do group 16 atoms have lower ionization energies than the corresponding group 15 atoms?
Glossary
- electron shielding: As more electrons are added to a nucleus, the outer electrons become shielded from the nucleus by the inner shell electrons.
- inner core electrons: Electrons that prevent attraction between valence electrons and protons.
Electron Affinity
Learning Objectives
- Define electron affinity.
- Describe trends in electron affinity in the periodic table.
Do you tend to overpack before going on trips?
Packing for a trip can be very challenging. What do you take with you? Where will you be going and what will you need? We usually pack too much (like the suitcase above) and then find it hard to close the suitcase. When the suitcase is over-full, there is stress on the system and forces pushing the suitcase open. When electrons are added to an atom, the increased negative charge puts stress on the electrons already there, causing energy to be released.
When electrons are removed from an atom, that process requires energy to pull the electron away from the nucleus. Addition of an electron releases energy from the process.
In most cases, the formation of an anion by the addition of an electron to a neutral atom releases energy. This can be shown for the chloride ion formation below:
Cl + e− → Cl− + energy
The energy change that occurs when a neutral atom gains an electron is called its electron affinity. When energy is released in a chemical reaction or process, that energy is expressed as a negative number. The figure below shows electron affinities in kJ/mole for the representative elements. Electron affinities are measured on atoms in the gaseous state and are very difficult to measure accurately.

Figure 25. Electron affinities (in kJ/mol) of the first five periods of the representative elements. Electron affinities are negative numbers because energy is released.
The elements of the halogen group (Group 17) gain electrons most readily, as can be seen from their large negative electron affinities. This means that more energy is released in the formation of a halide ion than for the anions of any other elements. Considering electron configuration, it is easy to see why. The outer configuration of all halogens is ns 2 np 5 . The addition of one more electron gives the halide ions the same electron configuration as a noble gas, which we have seen is particularly stable.
Period and group trends for electron affinities are not nearly as regular as for ionization energy. In general, electron affinities increase (become more negative) from left to right across a period and decrease (become less negative) from top to bottom down a group. However, there are many exceptions, owing in part to inherent difficulties in accurately measuring electron affinities.
Summary
- Electron affinity is a measure of the energy released when an extra electron is added to an atom.
- Electron affinities are measured in the gaseous state.
- In general, electron affinities become more negative as we move from left to right on the periodic table.
- In general, electron affinities become less negative from top to bottom of a group.
Practice
Use the link below to answer the following questions:
http://www.chemguide.co.uk/atoms/properties/eas.html
- Which groups (using the old Roman numeral system) of elements are primarily involved with issues of electron affinity?
- What does a negative energy imply?
- Why is the electron affinity value for fluorine less than that of chlorine?
Review
- Define “electron affinity.”
- Does addition of an electron to a neutral atom require energy or release energy?
- Describe the general trend for electron affinity values moving from left to right on the periodic table.
- Describe the general trend for electron affinity values moving from top to bottom in a group on the periodic table.
- Why is more energy released in the formation of a halide ion than with other elements?
Glossary
- electron affinity: The energy change that occurs when a neutral atom gains an electron.
Ionic Radii
Learning Objectives
- Define ionic radius.
- Explain how ionic radii are measured.
- Describes trends in ionic radius in the periodic table.
How are peanuts sold?

Peanuts can be sold two ways. The bare peanut without the shell (brown inner portion of peanut) can be purchased in jars and packages for casual munching or for cooking. The size of the peanut in this situation is smaller than the peanut plus shell since the outer portion is missing. If we add the shell to the peanut, we have a larger size for the combination.
Electrons and protons are strongly attracted to one another. The strength of that attraction and the relative numbers of the two particles in a given atom or ion have a significant influence on the size of that species. When an atom loses one or more electrons, the resulting ion becomes smaller. If electrons are added to the atom, the ion becomes larger.
The ionic radius for an atom is measured in a crystal lattice, requiring a solid form for the compound. These radii will differ somewhat depending upon the technique used. Usually X-ray crystallography is employed to determine the radius for an ion.

Figure 26. Comparison of ion sizes to atom sizes for Groups 1, 2, 13, 16 and 17. The atoms are shown in gray. Groups 1, 2, and 13 are metals and form cations, shown in red. Groups 16 and 17 are nonmetals and form anions, shown in blue.
The removal of electrons always results in a cation that is considerably smaller than the parent atom. When the valence electron(s) are removed, the resulting ion has one fewer occupied principal energy level, so the electron cloud that remains is smaller. Another reason is that the remaining electrons are drawn closer to the nucleus because the protons now outnumber the electrons. One other factor is the number of electrons removed. The potassium atom has one electron removed to for the corresponding ion, while calcium loses two electrons.
The addition of electrons always results in an anion that is larger than the parent atom. When the electrons outnumber the protons, the overall attractive force that the protons have for the electrons is decreased. The electron cloud also spreads out because more electrons results in greater electron-electron repulsions. Notice that the group 16 ions are larger than the group 17 ions. The group 16 elements each add two electrons while the group 17 elements add one electron per atom for form the anions.
Summary
- Ionic radius is determined by measuring the atom in a crystal lattice.
- Removal of electrons results in an ion that is smaller than the parent element.
- Addition of electrons results in an ion that is larger than the parent atom.
Practice
Use the link below to answer the following questions:
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch7/size.html
- What was the first compound used for determination of ionic radii?
- What assumptions were made in doing these measurements?
- Why is it important to know ionic radii?
Review
- How are ionic radii measured?
- Explain why the radius of the rubidium ion is smaller than the radius of the rubidium atom.
- Explain why the radius of the tellurium ion is larger than the radius of the tellurium atom.
- Why is the oxygen anion larger than the fluoride anion?
- Why is the sodium cation larger than the magnesium cation?
Glossary
- ionic radius: Determined by measuring the atom in a crystal lattice.
- crystal lattice: Inside a crystal, it’s the three-dimensional symmetrical arrangement of atoms.
Periodic Trends: Electronegativity
Learning Objectives
- Define electronegativity.
- Describe trends in electronegativity in the periodic table.
Is it easy or hard for you to make new friends?

Have you ever noticed how some people attract others to them? Whether it is their personality, attractiveness, or athletic skills – something pulls people toward them, while others have a smaller group of friends and acquaintances. Atoms do the same thing. One atom may pull electrons strongly to it, while a second type of atom has much less “pulling power.”
Valence electrons of both atoms are always involved when those two atoms come together to form a chemical bond. Chemical bonds are the basis for how elements combine with one another to form compounds. When these chemical bonds form, atoms of some elements have a greater ability to attract the valence electrons involved in the bond than other elements.
Electronegativity
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Electronegativity differs from electron affinity because electron affinity is the actual energy released when an atom gains an electron. Electronegativity is not measured in energy units, but is rather a relative scale. All elements are compared to one another, with the most electronegative element, fluorine, being assigned an electronegativity value of 3.98. Fluorine attracts electrons better than any other element. The table below shows the electronegativity values for the elements.

Figure 27. The electronegativity scale was developed by Nobel Prize winning American chemist Linus Pauling. The largest electronegativity (3.98) is assigned to fluorine and all other electronegativities measurements are on a relative scale.
Since metals have few valence electrons, they tend to increase their stability by losing electrons to become cations. Consequently, the electronegativities of metals are generally low. Nonmetals have more valence electrons and increase their stability by gaining electrons to become anions. The electronegativities of nonmetals are generally high.
Trends
Electronegativities generally increase from left to right across a period. This is due to an increase in nuclear charge. Alkali metals have the lowest electronegativities, while halogens have the highest. Because most noble gases do not form compounds, they do not have electronegativities. Note that there is little variation among the transition metals. Electronegativities generally decrease from top to bottom within a group due to the larger atomic size.
Of the main group elements, fluorine has the highest electronegativity (EN = 4.0) and cesium the lowest (EN = 0.79). This indicates that fluorine has a high tendency to gain electrons from other elements with lower electronegativities. We can use these values to predict what happens when certain elements combine. The following video shows this.
http://www.youtube.com/watch?v=Kj3o0XvhVqQ
When the difference between electronegativities is greater than ~1.7, then a complete exchange of electrons occurs. Typically this exchange is between a metal and a nonmetal. For instance, sodium and chlorine will typically combine to form a new compound and each ion becomes isoelectronic with its nearest noble gas. When we compare the EN values, we see that the electronegativity for Na is 0.93 and the value for Cl is 3.2. The absolute difference between ENs is |0.93 – 3.2| = 2.27. This value is greater than 1.7, and therefore indicates a complete electron exchange occurs.
Summary
- Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound.
- Electronegativity values generally increase from left to right across the periodic table.
- Electronegativities generally decrease from top to bottom of a group.
- The highest electronegativity value is for fluorine.
Practice
Use the link below to answer the following questions:
http://www.chemguide.co.uk/atoms/bonding/electroneg.html
- What are the least electronegative elements?
- What is a polar bond?
- What happens if atom A in a bond has much more electronegativity that atom B?
Review
- Define “electronegativity.”
- How does electronegativity differ from electron affinity?
- Why are the electronegativity values of metals generally low?
- Describe the trend in electronegativities across the periodic table.
- Describe the trends in electronegativities in a group of the periodic table.
Glossary
- electronegativity: A measure of the ability of an atom to attract the electrons when the atom is part of a compound.
Metallic and Nonmetallic Character
Learning Objectives
- Define metallic character.
- Define non-metallic character.
- Describe trends in metallic character in the periodic table.
- Describe trends in non-metallic character in the periodic table.
What are we eating as a nation?

The graph above indicates some trends in our diet over a thirty-year period. By observing the direction our eating habits are going, we can take steps to help prevent bad eating habits and decrease problems such as high blood pressure and heart attacks.
Development of the periodic table has helped organize chemical information in many ways. We can now see trends among properties of different atoms and make predictions about the behavior of specific materials.
Metallic character refers to the level of reactivity of a metal. Metals tend to lose electrons in chemical reactions, as indicated by their low ionization energies. Within a compound, metal atoms have relatively low attraction for electrons, as indicated by their low electronegativities. By following the trend summary in the figure below, you can see that the most reactive metals would reside in the lower left portion of the periodic table. The most reactive metal is cesium, which is not found in nature as a free element. It reacts explosively with water and will ignite spontaneously in air. Francium is below cesium in the alkali metal group, but is so rare that most of its properties have never been observed.

Figure 28. Trends in behaviors of elements.
Reactivity of metals is based on processes such as the formation of halide compounds with halogens and how easily they displace hydrogen from dilute acids.
The metallic character increases as you go down a group. Since the ionization energy decreases going down a group (or increases going up a group), the increased ability for metals lower in a group to lose electrons makes them more reactive. In addition, the atomic radius increases going down a group, placing the outer electrons further away from the nucleus and making that electron less attracted by the nucleus.
Nonmetals tend to gain electrons in chemical reactions and have a high attraction for electrons within a compound. The most reactive nonmetals reside in the upper right portion of the periodic table. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal. It is not found in nature as a free element. Fluorine gas reacts explosively with many other elements and compounds and is considered to be one of the most dangerous known substances.
Note that there is no clear division between metallic and non-metallic character. As we move across the periodic table, there is an increasing tendency to accept electrons (non-metallic) and a decrease in the possibility that an atom would give up one or more electrons.
Summary
- Metallic character refers to the level of reactivity of a metal.
- Non-metallic character relates to the tendency to accept electrons during chemical reactions.
- Metallic tendency increases going down a group.
- Non-metallic tendency increases going from left to right across the periodic table.
Practice
Read this page on periodic table trends to answer the following questions:
- List three characteristics of metals.
- List three characteristics of non-metals.
- Give two differences between metals and non-metals that would affect metallic and non-metallic properties.
Review
- Define “metallic character.”
- Define “non-metallic character.”
- Describe the trend in metallic character going down a group.
- Describe the trend in non-metallic character going across the periodic table.
- Why does the metallic character increase as you go down a group?
Glossary
- metallic: Refers to the level of reactivity of a metal.
- nonmetallic: Relates to the tendency to accept electrons during chemical reactions.
Candela Citations
- Chemistry Concepts Intermediate. Authored by: Calbreath, Baxter, et al.. Provided by: CK12.org. Located at: http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike