Learning Objectives
- Define Arrhenius acid.
- Give examples of Arrhenius acids.
- Describe the hydronium ion.
- Define Arrhenius base.
- Give examples of Arrhenius bases.
Arrhenius Acids
Don’t breathe too deeply.
 Venus is the planet nearest to us, but has a very different and hostile environment.
Venus is the planet nearest to us, but has a very different and hostile environment.
It has a surface temperature that averages around 450°C. The atmosphere is composed of carbon dioxide, but clouds of sulfuric acid move through the upper atmosphere, helping to create the extremely unfriendly conditions. Because of these conditions, Venus is not a place you want to visit on vacation.
Arrhenius Acids
Swedish chemist Svante Arrhenius (1859-1927) was the first to propose a theory to explain the observed behavior of acids and bases. Because of their ability to conduct a current, he knew that both acids and bases contained ions in solution. An Arrhenius acid is a compound, which ionizes to yield hydrogen ions (H + ) in aqueous solution.
Acids are molecular compounds with ionizable hydrogen atoms. Only hydrogen atoms that are part of a highly polar covalent bond are ionizable. Hydrogen chloride (HCl) is a gas at room temperature and under normal pressure. The H-Cl bond in hydrogen chloride is a polar bond. The hydrogen atom is electron deficient because of the higher electronegativity of the chlorine atom. Consequently, the hydrogen atom is attracted to the lone pair of electrons in a water molecule when HCl is dissolved in water. The result is that the H-Cl bond breaks, with both bonding electrons remaining with the Cl, forming a chloride ion. The H + ion attaches to the water molecule, forming a polyatomic ion called the hydronium ion. The hydronium ion (H 3 O + ) can be thought of as a water molecule with an attached hydrogen ion.

Figure 1. Formation of a hydronium ion.
Equations showing the ionization of an acid in water are frequently simplified by omitting the water molecule:
![]()
This is merely a simplification of the previous equation, but it is commonly used. Any hydrogen ions in an aqueous solution will be attached to water molecules as hydronium ions.
 Not all hydrogen atoms in molecular compounds are ionizable. In methane (CH 4 ), the hydrogen atoms are covalently bonded to carbon in bonds that are only slightly polar. The hydrogen atoms are not capable of ionizing and methane has no acidic properties. Acetic acid (CH 3 COOH) belongs to a class of acids called organic acids. There are four hydrogen atoms in the molecule, but only the one hydrogen that is bonded to an oxygen atom is ionizable.
Not all hydrogen atoms in molecular compounds are ionizable. In methane (CH 4 ), the hydrogen atoms are covalently bonded to carbon in bonds that are only slightly polar. The hydrogen atoms are not capable of ionizing and methane has no acidic properties. Acetic acid (CH 3 COOH) belongs to a class of acids called organic acids. There are four hydrogen atoms in the molecule, but only the one hydrogen that is bonded to an oxygen atom is ionizable.
The O-H bond can be ionized to yield the H + ion and the acetate ion. The other hydrogen atoms in this molecule are not acidic.
The Table below lists some of the more common acids:
| Acid Name | Formula |
| Hydrochloric acid | HCl |
| Nitric acid | HNO 3 |
| Sulfuric acid | H 2 SO 4 |
| Phosphoric acid | H 3 PO 4 |
| Acetic acid | CH 3 COOH |
| Hypochlorous acid | HClO |
A monoprotic acid is an acid that contains only one ionizable hydrogen. Hydrochloric acid and acetic acid are monoprotic acids. A polyprotic acid is an acid that contains multiple ionizable hydrogens. Most common polyprotic acids are either diprotic (such as H 2 SO 4 ) or triprotic (such as H 3 PO 4 ).
Arrhenius Bases
What can this be used for?

Sodium hydroxide is a versatile chemical. It can be used for such mundane purposes as cleaning clogged drains. Several commercial preparations contain sodium hydroxide for this purpose. It also has a number of applications in the food processing field. Ice cream is thickened using NaOH. If olives are soaked in a solution containing sodium hydroxide and other chemicals, the olives will turn black. Soft pretzels are made chewy by the application of sodium hydroxide to the food. This compound has been widely used in the synthesis of plastics, for etching aluminum, for paint removal, and is employed in the dehorning of cattle (in case that is a need you have).
Arrhenius Bases
An Arrhenius base is a compound, which ionizes to yield hydroxide ions (OH− ) in aqueous solution. The Table below lists several of the more common bases:
| Base Name | Formula |
| Sodium hydroxide | NaOH |
| Potassium hydroxide | KOH |
| Magnesium hydroxide | Mg(OH) 2 |
| Calcium hydroxide | Ca(OH) 2 |
All of the bases listed in the table are solids at room temperature. Upon dissolving in water, each dissociates into a metal cation and the hydroxide ion.
![]()
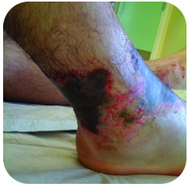
Figure 2. This foot has severe burns due to prolonged contact with a solution of sodium hydroxide, also known as lye.
Sodium hydroxide is a very caustic substance also known as lye. Lye is used as a rigorous cleaner and is an ingredient in the manufacture of soaps. Care must be taken with strong bases like sodium hydroxide, as exposure can lead to severe burns (see Figure 2 ).
Sodium belongs to the group of elements called the alkali metals. An alkaline solution is another name for a solution that is basic. All alkali metals react readily with water to produce the metal hydroxide and hydrogen gas. The resulting solutions are basic.
Bases that consist of an alkali metal cation and the hydroxide anion are all very soluble in water. Compounds of the Group 2 metals (the alkaline earth metals) are also basic. However, these compounds are generally not as soluble in water. Therefore the dissociation reactions for these compounds are shown as equilibrium reactions.
These relatively insoluble hydroxides were some of the compounds discussed in the context of the solubility product constant ![]() . The solubility of magnesium hydroxide is 0.0084 g per liter of water at 25°C. Because of its low solubility, magnesium hydroxide is not as dangerous as sodium hydroxide. In fact, magnesium hydroxide is the active ingredient in a product called milk of magnesia, which is used as an antacid or a mild laxative.
. The solubility of magnesium hydroxide is 0.0084 g per liter of water at 25°C. Because of its low solubility, magnesium hydroxide is not as dangerous as sodium hydroxide. In fact, magnesium hydroxide is the active ingredient in a product called milk of magnesia, which is used as an antacid or a mild laxative.
Summary
- Arrhenius acid is defined.
- Examples of Arrhenius acids are given.
- Arrhenius base is defined.
- Examples of Arrhenius bases are given.
Practice
Read the material at the link below and answer the following questions:
- What was Arrhenius’ first scientific idea?
- What did his Ph.D committee think about it?
- What did he win for this idea?
- Write the generic equation for dissociation of an Arrhenius acid.
Review
Arrhenius Acids
- What is an Arrhenius acid?
- What is a hydronium ion?
- Is H2SO4 a monoprotic or a polyprotic acid?
Arrhenius Bases
- What is an Arrhenius base?
- What is one reaction that will form an Arrhenius base?
- Are alkaline earth bases very water-soluble?
Glossary
- Arrhenius acid: A compound which ionizes to yield hydrogen ions (H+) in aqueous solution.
- hydronium ion (H3O+): A water molecule with an attached hydrogen ion.
- monoprotic acid: An acid that contains only one ionizable hydrogen.
- polyprotic acid: An acid that contains multiple ionizable hydrogens.
- alkaline solution: Another name for a solution that is basic.
- Arrhenius base: A compound which ionizes to yield hydroxide ions (OH−) in aqueous solution.