Learning Objectives
- Define kinetic energy.
- Describe how temperature influences kinetic energy of particles.
- Define absolute zero.
Examples

How much energy does it take to hit a baseball?
Kinetic energy is the energy of motion. Any object that is moving possesses kinetic energy. Baseball involves a great deal of kinetic energy. The pitcher throws a ball, imparting kinetic energy to the ball. When the batter swings, the motion of swinging creates kinetic energy in the bat. The collision of the bat with the ball changes the direction and speed of the ball, with the idea of kinetic energy being involved again.
Kinetic Energy and Temperature
As stated in the kinetic-molecular theory, the temperature of a substance is related to the average kinetic energy of the particles of that substance. When a substance is heated, some of the absorbed energy is stored within the particles, while some of the energy increases the motion of the particles. This is registered as an increase in the temperature of the substance.
Average Kinetic Energy
At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Instead, the particles display a wide range of kinetic energies. Most of the particles have a kinetic energy near the middle of the range. However, a small number of particles have kinetic energies a great deal lower or a great deal higher than the average (see Figure below ).
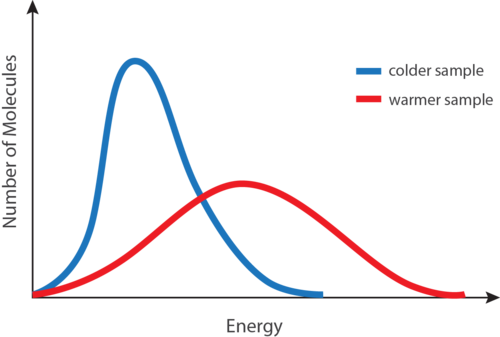
Figure 13.5
A distribution of molecular kinetic energies as a function of temperature. The blue curve is for a low temperature, while the red curve is for a high temperature.
The blue curve in the figure above is for a sample of matter at a relatively low temperature, while the red curve is for a sample at a relatively high temperature. In both cases, most of the particles have intermediate kinetic energies, close to the average. Notice that as temperature increases, the range of kinetic energies increases and the distribution curve “flattens out.” At a given temperature, the particles of any substance have the same average kinetic energy.
Absolute Zero
As a sample of matter is continually cooled, the average kinetic energy of its particles decreases. Eventually, one would expect the particles to stop moving completely. Absolute zero is the temperature at which the motion of particles theoretically ceases. Absolute zero has never been attained in the laboratory, but temperatures on the order of 1 × 10 -10 K have been achieved. TheKelvin temperature scale is the scale that is based on molecular motion and so absolute zero is also called 0 K. The Kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. For example, the particles in a sample of hydrogen gas at 200 K have twice the average kinetic energy as the particles in a hydrogen sample at 100 K.

Figure 13.6
Helium gas liquefies at 4 K or four degrees above absolute zero. Liquid helium is used as a coolant for large superconducting magnets and must be stored in insulated metal canisters.
Summary
- Kinetic energy is the energy of motion.
- At a given temperature, individual particles of a substance have a range of kinetic energies.
- The motion of particles theoretically ceases at absolute zero.
Practice
Watch the presentation and take the quiz at the end:
https://www.wisc-online.com/learn/natural-science/chemistry/gch4904/the-kinetic-theory-of-gases
Review
- What is kinetic energy?
- If the temperature increases, will particles move faster or slower that they would ata lower temperature?
- What is absolute zero?
- List one use for liquid helium.
Glossary
- absolute zero: The temperature at which the motion of particles theoretically ceases.
- Kelvin scale: The scale that is based on molecular motion and so absolute zero is also called 0 K.
- kinetic energy: The energy of motion.