Learning Objectives
- Perform calculations involving the effects of electrolytes on colligative properties.
What effect do ions have?

Ionic solution measurement. Image from Wikimedia.
The addition of ions creates significant changes in properties of solutions. Water molecules surround the ions and are somewhat tightly bound to them. Colligative properties are affected because the solvent properties are no longer the same as those in the pure solvent.
Electrolytes and Colligative Properties
Ionic compounds are electrolytes and dissociate into two or more ions as they dissolve. This must be taken into account when calculating the freezing and boiling points of electrolyte solutions. The sample problem below demonstrates how to calculate the freezing point and boiling point of a solution of calcium chloride. Calcium chloride dissociates into three ions according to the equation:
![]()
The values of the freezing point depression and the boiling point elevation for a solution of CaCl 2 will be three times greater than they would be for an equal molality of a nonelectrolyte.
Sample Problem: Freezing and Boiling Point of an Electrolyte
Determine the freezing and boiling point of a solution prepared by dissolving 82.20 g of calcium chloride into 400.g of water.
Step 1: List the known quantities and plan the problem.
Known
- mass CaCl 2 = 82.20 g
- molar mass CaCl 2 = 110.98 g/mol
- mass H 2 O = 400. g = 0.400 kg


- CaCl 2 dissociates into 3 ions
Unknown
The moles of CaCl 2 is first calculated, followed by the molality of the solution. The freezing and boiling points are then determined, including multiplying by 3 for the three ions.
Step 2: Solve.
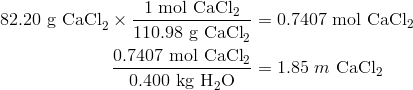
![]()
Step 3: Think about your result.
Since the normal boiling point of water is 100.00°C, the calculated result for ![]() must be added to 100.00 to find the new boiling point.
must be added to 100.00 to find the new boiling point.
Summary
- The effect of ionization on colligative properties is described.
Practice
Do the practice and homework problems dealing with ionic solutions toward the end of the section on the link below:
Review
- Why do ionic materials change the colligative properties of a solution?
- Would HCl be expected to alter colligative properties?
- Calcium carbonate is ionic, but insoluble in water. What effect would it have on the boiling point of water?
Candela Citations
- Chemistry Concepts Intermediate. Authored by: Calbreath, Baxter, et al.. Provided by: CK12.org. Located at: http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/. License: CC BY-NC: Attribution-NonCommercial