Learning Objectives
- Define phase diagram.
- Define the triple point.
- Describe how to use the phase diagram to indicate the state of a material and different temperatures and pressures.
Examples

How are rockets able to shoot up into the air?
Many rockets use a combination of kerosene and liquid oxygen for their fuel. Oxygen can be reduced to the liquid state either by cooling or by using high pressure. Since the oxygen is in a container essentially out in the open, maintaining a temperature of -183°C (the boiling point of oxygen) is not real practical. But high pressure can be used to force the oxygen into tanks and cause it to liquefy so it can then mix with the kerosene and provide a powerful ignition to move the rocket.
Phase Diagrams
The relationships among the solid, liquid, and vapor (gas) states of a substance can be shown as a function of temperature and pressure in single diagram. A phase diagram is graph showing the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas phases. Examine the general phase diagram shown in the Figure below . In each of the three colored regions of the diagram, the substance is in a single state (or phase). The dark lines that act as the boundary between those regions represent the conditions under which the two phases are in equilibrium.

Figure 13.25
General phase diagram, which shows the state (phase) of a substance as a function of its temperature and pressure.
Find the ![]() on the pressure axis and presume that the value of
on the pressure axis and presume that the value of ![]() is standard pressure of 1 atm. As one moves left to right across the red line, the temperature of the solid substance is being increased while the temperature remains constant. When point
is standard pressure of 1 atm. As one moves left to right across the red line, the temperature of the solid substance is being increased while the temperature remains constant. When point ![]() is reached, the substance melts and the temperature
is reached, the substance melts and the temperature ![]() on the horizontal axis represents the normal melting point of the substance. Moving further to the right, the substance boils at point
on the horizontal axis represents the normal melting point of the substance. Moving further to the right, the substance boils at point ![]() and so point
and so point ![]() on the horizontal axis represents the normal boiling point of the substance. As the temperature increases at a constant pressure, the substance changes from solid to liquid to gas.
on the horizontal axis represents the normal boiling point of the substance. As the temperature increases at a constant pressure, the substance changes from solid to liquid to gas.
Start right above point ![]() on the temperature axis and follow the red line vertically. At very low pressure, the particles of the substance are far apart from one another and the substance is in the gas state. As the pressure is increased, the particles of the substance are forced closer and closer together. Eventually the particles are pushed so close together that attractive forces cause the substance to condense into the liquid state. Continually increasing the pressure on the liquid will eventually cause the substance to solidify. For the majority of substances, the solid state is denser than the liquid state and so putting a liquid under great pressure will cause it to turn into a solid. The line segment
on the temperature axis and follow the red line vertically. At very low pressure, the particles of the substance are far apart from one another and the substance is in the gas state. As the pressure is increased, the particles of the substance are forced closer and closer together. Eventually the particles are pushed so close together that attractive forces cause the substance to condense into the liquid state. Continually increasing the pressure on the liquid will eventually cause the substance to solidify. For the majority of substances, the solid state is denser than the liquid state and so putting a liquid under great pressure will cause it to turn into a solid. The line segment ![]() represents the process of sublimation, where the substance changes directly from a solid to a gas. At a sufficiently low pressure, the liquid phase does not exist. The point labeled
represents the process of sublimation, where the substance changes directly from a solid to a gas. At a sufficiently low pressure, the liquid phase does not exist. The point labeled ![]() is called the triple point . The triple point is the one condition of temperature and pressure where the solid, liquid, and vapor states of a substance can all coexist at equilibrium.
is called the triple point . The triple point is the one condition of temperature and pressure where the solid, liquid, and vapor states of a substance can all coexist at equilibrium.
Summary
- A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas.
- The triple point is the one condition of temperature and pressure where the solid, liquid, and vapor states of a substance can all coexist at equilibrium.
Practice
Use the link below to answer the following questions:
- What temperatures and pressures favor the formation of a solid?
- What temperatures and pressures favor the formation of a gas?
- What does the line
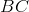 represent?
represent? - What does the line
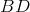 represent?
represent?
Review
- What is a phase diagram?
- What is the triple point?
- What does point
 represent?
represent?
Glossary
- phase diagram: A graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas.
- triple point: The one condition of temperature and pressure where the solid, liquid, and vapor states of a substance can all coexist at equilibrium.