Learning Objectives
- Describe molecules whose shapes are influenced by VSEPR theory.
- Define lone pair.
- Describe how lone pair electrons influence molecular geometry.
How does an electroscope work?
An electroscope is a device used to study charge. When a positively charged object (the rod) nears the upper post, electrons flow to the top of the jar leaving the two gold leaves postivley charged. The leaves repel each other since both hold postive, like charges. The VSEPR theory says that electron pairs, also a set of like charges, will repel each other such that the shape of the molecule will adjust so that the valence electron-pairs stay as far apart from each other as possible.

Central Atom with No Lone Pairs
In order to easily understand the types of molecules possible, we will use a simple system to identify the parts of any molecule.
A = central atom in a molecule
B = atoms surrounding the central atom
Subscripts after the B will denote the number of B atoms that are bonded to the central A atom. For example, AB 4 is a molecule with a central atom surrounded by four covalently bonded atoms. Again, it does not matter if those bonds are single, double, or triple bonds.
AB2: Beryllium hydride (BeH2)
Beryllium hydride consists of a central beryllium atom with two single bonds to hydrogen atoms. Recall that it violates the octet rule.
H-Be-H
According to the requirement that electron pairs maximize their distance from one another, the two bonding pairs in the BeH 2 molecules will arrange themselves on directly opposite sides of the central Be atom. The resulting geometry is a linear molecule, shown in the Figure 1 in a “ball and stick” model.

Figure 1. Beryllium hydride model.
The bond angle from H-Be-H is 180° because of its linear geometry.
Carbon dioxide is another example of a molecule which falls under the AB 2 category. Its Lewis structure consists of double bonds between the central carbon and the oxygen atoms (see Figure 2).

Figure 2. Carbon dioxide bonding.
The repulsion between the two groups of four electrons (two pairs) is no different than the repulsion of the two groups of two electrons (one pair) in the BeH 2 molecule. Carbon dioxide is also linear (see Figure 3).

Figure 3. Carbon dioxide.
AB3: Boron Trifluoride (BF3)
Boron trifluoride consists of a central boron atom with three single bonds to fluorine atoms (see Figure 4). The boron atom also has an incomplete octet.
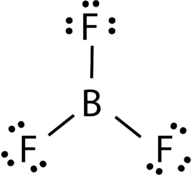
Figure 4. Boron trifluoride bonding.
The geometry of the BF 3 molecule is called trigonal planar (see Figure 5). The fluorine atoms are positioned at the vertices of an equilateral triangle. The F-B-F angle is 120° and all four atoms lie in the same plane.

Figure 5. Boron trifluoride model.
AB4: Methane (CH4)
Methane is an organic compound that is the primary component of natural gas. Its structure consists of a central carbon atom with four single bonds to hydrogen atoms (see Figure 6). In order to maximize their distance from one another, the four groups of bonding electrons do not lie in the same plane. Instead, each of the hydrogen atoms lies at the corners of a geometrical shape called a tetrahedron. The carbon atom is at the center of the tetrahedron. Each face of a tetrahedron is an equilateral triangle.

Figure 6. Tetrahedral structure of methane.
The molecular geometry of the methane molecule is tetrahedral (see Figure 7). The H-C-H bond angles are 109.5°, which is larger than the 90° that they would be if the molecule was planar. When drawing a structural formula for a molecule such as methane, it is advantageous to be able to indicate the three-dimensional character of its shape. The structural formula below is called a perspective drawing. The dotted line bond is to be visualized as receding into the page, while the solid triangle bond is to be visualized as coming out of the page.

Figure 7. Methane perspective model.
How can all these clothes fit into such a small space?
When we travel, we often take a lot more stuff than we need. Trying to fit it all in a suitcase can be a real challenge. We may have to repack or just squeeze it all in. Atoms often have to rearrange where the electrons are in order to create a more stable structure.

Central Atom with One or More Lone Pairs
The molecular geometries of molecules change when the central atom has one or more lone pairs of electrons. The total number of electron pairs, both bonding pairs and lone pairs, leads to what is called the electron domain geometry. When one or more of the bonding pairs of electrons is replaced with a lone pair, the molecular geometry (actual shape) of the molecule is altered. In keeping with the A and B symbols established in the previous section, we will use E to represent a lone pair on the central atom (A). A subscript will be used when there is more than one lone pair. Lone pairs on the surrounding atoms (B) do not affect the geometry.
AB3E: Ammonia, NH3
The ammonia molecule contains three single bonds and one lone pair on the central nitrogen atom (see Figure 8).

Figure 8. Lone pair electrons in ammonia.
The domain geometry for a molecule with four electron pairs is tetrahedral, as was seen with CH 4 . In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. The molecular geometry of NH 3 is called trigonal pyramidal (see Figure 9).

Figure 9. Ammonia molecule.
Recall that the bond angle in the tetrahedral CH 4 molecule is 109.5°. Again, the replacement of one of the bonded electron pairs with a lone pair compresses the angle slightly. The H-N-H angle is approximately 107°.
AB2E2: Water, H2O
A water molecule consists of two bonding pairs and two lone pairs (see Figure 10).

Figure 10. Lone pair electrons on water.
As for methane and ammonia, the domain geometry for a molecule with four electron pairs is tetrahedral. In the water molecule, two of the electron pairs are lone pairs rather than bonding pairs. The molecular geometry of the water molecule is bent. The H-O-H bond angle is 104.5°, which is smaller than the bond angle in NH3 (see Figure 11).

Figure 11. Water molecule.
AB4E: Sulfur Tetrafluoride, SF4
The Lewis structure for SF 4 contains four single bonds and a lone pair on the sulfur atom (see Figure 12).

Figure 12. Lone pair electrons in SF4.
The sulfur atom has five electron groups around it, which corresponds to the trigonal bipyramidal domain geometry, as in PCl 5 (see Figure 13). Recall that the trigonal bipyramidal geometry has three equatorial atoms and two axial atoms attached to the central atom. Because of the greater repulsion of a lone pair, it is one of the equatorial atoms that are replaced by a lone pair. The geometry of the molecule is called a distorted tetrahedron or seesaw.

Figure 13. Ball and stick model for SF4 .
| Total Number of Electron Pairs | Number of Bonding Pairs | Number of Lone Pairs | Electron Domain Geometry | Molecular Geometry | Examples |
| 3 | 2 | 1 | trigonal planar | bent | O3 |
| 4 | 3 | 1 | tetrahedral | trigonal pyramidal | NH3 |
| 4 | 2 | 2 | tetrahedral | bent | H2O |
| 5 | 4 | 1 | trigonal bipyramidal | distorted tetrahedron (seesaw) | SF4 |
| 5 | 3 | 2 | trigonal bipyramidal | T-shaped | CIF3 |
| 5 | 2 | 3 | trigonal bipyramidal | linear | I3– |
| 6 | 5 | 1 | octahedral | square pyramidal | BrF5 |
| 6 | 4 | 2 | octahedral | square planar | XeF4 |
Summary
- Electron pairs repel each other and influence bond angles and molecular shape.
- The presence of lone pair electrons influences the three-dimensional shape of the molecule.
Practice
Central Atom with No Lone Pairs
Use the link below to answer the following questions:
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/vsepr.html
- What is the shape of PF 5 ?
- What C-H bod angles would we predict for methane if the molecule were planar?
- What molecule has the configuration of an octahedron?
Central Atom with One or More Lone Pairs
Use the link below to answer the following questions:
- What is the general principle in dealing with molecules containing more than four electron pairs?
- In the picture with five electron pairs around the central atom, why is the arrangement on the right preferred?
- In the picture with six electron pairs, why is the configuration with the lone pairs at 180o to each other more stable?
Review
Central Atom with No Lone Pairs
- What are the bond angles in carbon dioxide?
- What molecule has bond angles of 109.5 ° ?
- What is the geometry of the BF 3 molecule?
Central Atom with One or More Lone Pairs
- Why does water have a bent geometry?
- Why is ammonia not a planar molecule?
- How would we write the configuration for xenon tetrafluoride using the ABE system?
Glossary
- central atom: The atom around which other atoms are arranged.
- electron domain geometry: Geometry based only on the number of electron pairs around the central atom, both bonding pairs and lone pairs.
Candela Citations
- Chemistry Concepts Intermediate. Authored by: Calbreath, Baxter, et al.. Provided by: CK12.org. Located at: http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/. License: CC BY-NC: Attribution-NonCommercial