Learning Objectives
- Explain genetic studies, twin and adoption research, and biological explanations for schizophrenia
Causes of Schizophrenia
Genes

Figure 1. Twin and adoptions studies estimate that the heritability of schizophrenia may be up to 80%.
When considering the role of genetics in schizophrenia, as in any disorder, conclusions based on family and twin studies have some inherent flaws because family members who are closely related (such as siblings) are more likely to share similar environments than are family members who are less closely related (such as cousins); further, identical twins may be more likely to be treated similarly by others than might fraternal twins. Thus, family and twin studies cannot completely rule out the possible effects of shared environments and experiences. Such problems can be corrected by using adoption studies, in which children are separated from their parents at an early age. One of the first adoption studies of schizophrenia conducted by Heston (1966) followed 97 adoptees, including 47 who were born to mothers with schizophrenia, over a 36-year period. Five of the 47 adoptees (11%) whose mothers had schizophrenia were later diagnosed with schizophrenia, compared to none of the 50 control adoptees. Other adoption studies have consistently reported that for adoptees who are later diagnosed with schizophrenia, their biological relatives have a higher risk of schizophrenia than do adoptive relatives (Shih, Belmonte, & Zandi, 2004).
Individual twin studies and meta-analyses of twin studies have estimated the heritability of risk for schizophrenia to be approximately 80% (this refers to the proportion of variation between individuals in a population that is influenced by genetic factors, not the degree of genetic determination of individual risk), but the heritability estimate varies from 41 to 87%. Concordance rates (the percentage of twins whose co-twin has the same condition) between monozygotic twins vary in different studies, with an average estimate of approximately 50%; whereas dizygotic twins was 17%. Some twin studies have found rates as low as 11.0%–13.8% among monozygotic twins, and 1.8%–4.1% among dizygotic twins, however. Genetics do appear to play a strong role in the etiology of schizophrenia, but others factors are also clearly involved as predicted by the biopsychosocial model.
Genetics and Schizophrenia
Read this explanatory message, “Piecing Together the Genetic Puzzle of Schizophrenia,” by Joshua Gordon, the director of the National Institute of Mental Health. Remember that genes may interact with each other, some of them suppressing other genes’ activity and others enhancing them; epigenetic research also teaches us that some genes also interact with the environment, and that interaction shapes the outcomes of the genes’ functions. Also, everyone (with the exception of twins) has their own genome; out of the many genetic variations involved in schizophrenia, a given person may inherit only a portion of the problematic genes. This complex situation may help us understand why many experts consider schizophrenia, other psychotic disorders, and even personality disorders, such as schizoid or schizotypal personality disorders as part of a family or spectrum, a range of disorders that may all relate back to hundreds of interacting genetic variations. No two people have the same symptoms even if they have the same schizophrenia diagnosis.
Genetics does play a substantial role in the origins of schizophrenia, a role that is both pretty straightforward and incredibly complicated. The straightforward part: genetic differences account for a significant portion of our risk of having a mental illness. The complicated part: determining how these genetic differences contribute to risk is anything but straightforward.
One way to think about it is to consider two very different kinds of genetic traits: (red) hair color and height. Both run in families, but in very different ways. These differences can help us think about the kinds of genetic differences that underlie schizophrenia.
Most people with red hair have it because of a known alteration, or mutation, in the gene for a protein called the melanocortin-1 receptor (MC1R). If you have one or two copies of the unaltered MC1R gene, you will have brown or black hair. But if you have two copies of the altered gene, you’ll have red hair. There are some exceptions to this rule, but, for the vast majority of people, this one gene determines whether they have red hair or not.
The story is very different for height. According to the National Human Genome Research Institute, there are more than 700 genes that can contribute to determining how tall a person will be. It’s the combined action of some of these individual genetic differences, interacting with environmental factors (epigenetics) such as diet and exercise, that influence how tall someone is likely to be when fully grown.
It has become clear that the genetics of schizophrenia is less like that of red hair and more like that of height. Over the past six years, NIMH-funded investigators and collaborators around the globe have amassed an impressively large genetics dataset, thanks to the generous participation of more than 60,000 individuals with schizophrenia and almost 100,000 individuals without the disorder. Comparing data from individuals in these two groups, scientists have identified over 250 places in the genome that contribute to the overall risk for schizophrenia. As with height, each one of these genetic risk factors plays a small role in influencing individual risk. Collectively, however, they play a significant role.
These findings are really good news, as each of these 250 risk factors represents an important clue into the biology of schizophrenia. However, the sheer number of risk factors, plus the fact that we still don’t have a clear understanding of how these risk factors interact at the individual level, poses a challenge for those of us who would like to translate these genetic findings into better treatments. How can we study the relevant effects of these genes when we don’t know which combinations are important? Although we have some ideas about how to proceed — for example, we can look for common biological mechanisms shared by multiple genes — it will take a while for us to sort out the best way to learn about schizophrenia from these “small-effect-size” variations.
But is schizophrenia all “small-effect-size” variations? In the past few months, researchers have started to find some rare, larger effect-size differences in single genes using a method called whole-exome sequencing.
Whole-exome sequencing involves reading the DNA sequences of all “protein-coding” genes — that is, all of the genetic information that gets transcribed into messenger RNA and used to make proteins. The exome is a prime target for investigation because we have a good understanding of how the genetic code is translated into proteins and, most importantly, how changes in protein structure can lead to dysfunction. This allows scientists to home in on the genetic differences that are likely to impact the function of proteins and cause disease.
With support from NIMH and other research funding organizations, psychiatric geneticists have been able to sequence the exomes of thousands of people with schizophrenia, and the effort is starting to pay off. A collaborative effort led by Ben Neale, Ph.D., a genetics researcher at the Broad Institute of MIT and Harvard and Massachusetts General Hospital, examined data from more than 2,700 people with schizophrenia and found the strongest evidence to date that rare genetic mutations increase the likelihood of developing schizophrenia. The findings, recently published in Nature Neuroscience, add to unpublished evidence presented at the most recent meeting of the World Congress of Psychiatric Genetics. Presenting on behalf of the SCHEMA (Schizophrenia Exome Meta-Analysis) consortium, Broad Institute researcher Tarjinder Singh, Ph.D., reported findings from a larger exome sequencing study that found several rare genetic variants with large-effect-size changes.
None of these gene variants will lead to schizophrenia in the way that the MC1R variation leads to red hair — an individual’s risk of having schizophrenia is influenced by the combination of many genetic differences with small and large effects, all working in concert. Nonetheless, these whole-exome sequencing findings are game-changing. Why? One reason is that it is much more straightforward to study the biological mechanisms underlying large-effect-size genetic differences in protein-coding genes — researchers can learn about the effects by introducing single gene mutations in human cell lines or model organisms and then looking for changes in molecular and biological processes.
In addition, the biological pathways affected by large-effect-size variations can suggest novel therapeutic targets. One quick example — two of the genes identified in the research presented at the World Congress of Psychiatric Genetics are known to code for receptors for the neurotransmitter glutamate. Researchers had previously hypothesized that drugs affecting the glutamate system may be useful in the treatment of schizophrenia; these new findings give greater credence and specificity to efforts to develop glutamatergic agents as novel therapeutics.
Although adoption studies have supported the hypothesis that genetic factors contribute to schizophrenia, they have also demonstrated that the disorder most likely arises from a combination of genetic and environmental factors rather than just genes themselves. For example, investigators in one study examined the rates of schizophrenia among 303 adoptees (Tienari et al., 2004). A total of 145 of the adoptees had biological mothers with schizophrenia; these adoptees constituted the high genetic risk group. The other 158 adoptees had mothers with no psychiatric history; these adoptees composed the low genetic risk group. The researchers managed to determine whether the adoptees’ families were either healthy or disturbed. The adoptees were considered to be raised in a disturbed family environment if the family exhibited a lot of criticism, conflict, and a lack of problem-solving skills. The findings revealed that adoptees whose mothers had schizophrenia (high genetic risk) and who had been raised in a disturbed family environment were much more likely to develop schizophrenia or another psychotic disorder (36.8%) than were adoptees whose biological mothers had schizophrenia but who had been raised in a healthy environment (5.8%), or than adoptees with a low genetic risk who were raised in either a disturbed (5.3%) or healthy (4.8%) environment. Since the adoptees who were at high genetic risk were likely to develop schizophrenia only if they were raised in a disturbed home environment, this study supports a biopsychosocial diathesis-stress interpretation of schizophrenia—both genetic vulnerability and environmental stress are necessary for schizophrenia to develop; genes alone do not show the complete picture.
The diathesis-stress model of schizophrenia explains how an environmental trigger, such as severe stress or drug use, can catalyze the onset of a disease in a genetically susceptible person. This model does not mean that without the specific environmental trigger the person would be safe from developing schizophrenia, but it does display the importance of the environment on genetic expression. For example, a young woman in her early to mid-twenties who has a genetic propensity to develop schizophrenia can go through a traumatic relationship, and that experience then catalyzes the initial onset of schizophrenic symptoms known as the prodromal phase.
Neurotransmitters
If we accept that schizophrenia is at least partly genetic in origin, as it seems to be, it makes sense that the next step should be to identify biological abnormalities commonly found in people with the disorder. Perhaps not surprisingly, a number of neurobiological factors have indeed been found to be related to schizophrenia. One such factor that has received considerable attention for many years is the neurotransmitter dopamine. Interest in the role of dopamine in schizophrenia was stimulated by two sets of findings: drugs that increase dopamine levels can produce schizophrenia-like symptoms and medications that block dopamine activity reduce positive symptoms (Howes & Kapur, 2009). These findings were the oldest biologically focused hypothesis about the etiology of schizophrenia that began with research first conducted in the 1950s.
The dopamine hypothesis of schizophrenia proposed that an overabundance of dopamine or too many dopamine receptors are responsible for the onset and maintenance of schizophrenia (Snyder, 1976). More recent work in this area suggests that abnormalities in dopamine vary by brain region and thus contribute to symptoms in unique ways. In general, this research has suggested that an overabundance of dopamine in the limbic system may be responsible for some symptoms, such as hallucinations and delusions, whereas low levels of dopamine in the prefrontal cortex might be responsible primarily for the negative symptoms (avolition, alogia, asociality, and anhedonia) (Davis, Kahn, Ko, & Davidson, 1991). In recent years, serotonin has received attention and newer antipsychotic medications used to treat the disorder work by blocking serotonin receptors (Baumeister & Hawkins, 2004). As noted above in the summary by Dr. Gordon, large effect-size variations studies are now also suggesting an important role for the neurotransmitter glutamate.
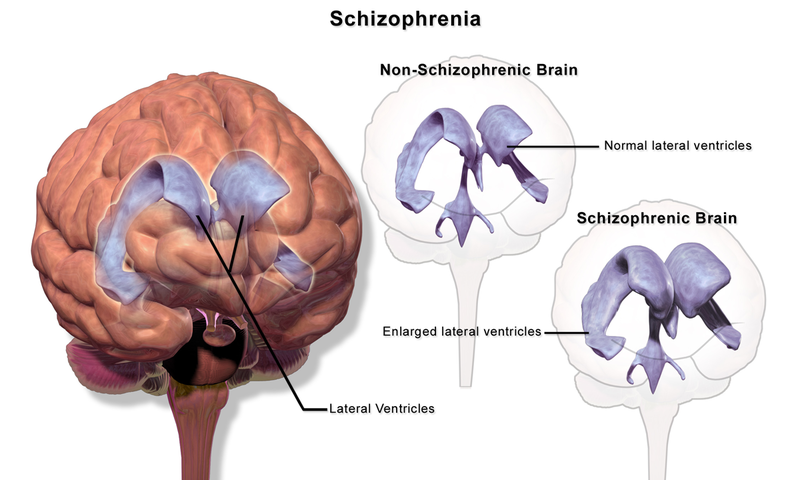
Brain Anatomy
Brain imaging studies reveal that people with schizophrenia have enlarged ventricles, the cavities within the brain that contain cerebral spinal fluid (Green, 2001). This finding is important because larger than normal ventricles suggests that various brain regions are reduced in size, thus implying that schizophrenia is associated with a loss of brain tissue. In addition, many people with schizophrenia display a reduction in gray matter (cell bodies of neurons) in the frontal lobes (Lawrie & Abukmeil, 1998), and many show less frontal lobe activity when performing cognitive tasks (Buchsbaum et al., 1990). The frontal lobes are important in a variety of complex cognitive functions, such as planning and executing behavior, attention, speech, movement, and problem-solving. Hence, abnormalities in this region provide merit in explaining why people with schizophrenia experience deficits in these areas. We do not yet understand the mechanisms or processes involved in the apparent atrophy of brain tissue in the brains of persons with schizophrenia or when this process takes place as a person grows and matures.
The Biological Perspective: Neuroimaging
The advent of neuroimaging techniques like structural and functional magnetic resonance imaging and positron emission tomography opened up the ability to try to understand the brain mechanisms of the symptoms of schizophrenia as well as the cognitive impairments found in psychosis. For example, a number of studies have suggested that delusions in psychosis may be associated with problems in salience detection mechanisms supported by the ventral striatum (Jensen & Kapur, 2009; Jensen et al., 2008; Kapur, 2003; Kapur, Mizrahi, & Li, 2005; Murray et al., 2008) and the anterior prefrontal cortex (Corlett et al., 2006; Corlett, Honey, & Fletcher, 2007; Corlett, Murray, et al., 2007a, 2007b). These are regions of the brain that normally increase their activity when something important (a.k.a. salient) happens in the environment. If these brain regions misfire, individuals with psychosis may mistakenly attribute importance to irrelevant or unconnected events.
Further, there is good evidence that problems in working memory and cognitive control in schizophrenia are related to problems in the function of a region of the brain called the dorsolateral prefrontal cortex (DLPFC) (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009; Ragland et al., 2009). These problems include changes in how the dorsolateral prefrontal cortex (DLPFC) works when people are doing working-memory or cognitive-control tasks, and problems with how this brain region is connected to other brain regions important for working memory and cognitive control, including the posterior parietal cortex (e.g., Karlsgodt et al., 2008; J. J. Kim et al., 2003; Schlosser et al., 2003), the anterior cingulate (Repovs & Barch, 2012), and temporal cortex (e.g., Fletcher et al., 1995; Meyer-Lindenberg et al., 2001).
In terms of understanding episodic memory problems in schizophrenia, many researchers have focused on medial temporal lobe deficits, with a specific focus on the hippocampus (e.g., Heckers & Konradi, 2010) because there is much data from humans and animals showing that the hippocampus is important for the creation of new memories (Squire, 1992). However, it has become increasingly clear that problems with the dorsolateral prefrontal cortex (DLPFC) also make important contributions to episodic memory deficits in schizophrenia (Ragland et al., 2009), probably because this part of the brain is important for controlling our use of memory.
In addition to problems with regions such as the DLFPC and medial temporal lobes in schizophrenia described above, magnitude resonance neuroimaging studies have also identified changes in cellular architecture, white matter connectivity, and gray matter volume in a variety of regions that include the prefrontal and temporal cortices (Bora et al., 2011). People with schizophrenia also show reduced overall brain volume. Reductions in brain volume as people get older may be larger in those with schizophrenia than in healthy people (Olabi et al., 2011). Taking antipsychotic medications or using drugs such as marijuana, alcohol, and tobacco may cause some of these structural changes. However, these structural changes are not completely explained by medications or substance use alone. Further, both functional and structural brain changes are seen, again to a milder degree, in the first-degree relatives of people with schizophrenia (Boos, Aleman, Cahn, Pol, & Kahn, 2007; Brans et al., 2008; Fusar-Poli et al., 2007; MacDonald, Thermenos, Barch, & Seidman, 2009), again suggesting that neural changes associated with schizophrenia are related to a genetic risk for this illness.
Environmental Risk Factors
As mentioned previously, some environmental factors are associated with a slight risk of developing schizophrenia in later life, such as concerns during prenatal development, including oxygen deprivation, infection, prenatal maternal stress, and malnutrition. Some studies found that the mother having the flu during the first or second trimesters increases the risk of a child having schizophrenia, but those results have been questioned.[1] Both maternal stress and infection have been demonstrated to alter fetal neurodevelopment through an increase of pro-inflammatory cytokines. There is a slighter higher risk associated with being born in the winter or spring possibly due to vitamin D deficiency or a prenatal viral infection. Other infections during pregnancy or around the time of birth that have been linked to an increased risk include infections by toxoplasma gondii and chlamydia. Viral infections of the brain during childhood are also linked to a risk of schizophrenia during adulthood.
Living in an urban environment during childhood or as an adult has consistently been found to increase the risk of schizophrenia by a factor of two, even after taking into account drug use, ethnic group, and size of social group. A possible link between the urban environment and pollution has been suggested to be the cause of the elevated risk of schizophrenia.
Other risk factors of importance include social isolation, immigration related to social adversity and racial discrimination, family dysfunction, unemployment, and poor housing conditions. Having a father older than 40 years old or parents younger than 20 years old are also associated with schizophrenia. It has been suggested that apart from gene-environment interactions, environment-environment interactions also be taken into account as each environmental risk factor on its own is not enough.
Link to Learning
This PBS News Hour clip highlights some research conducted by Steven McCarroll, an associate professor of genetics at Harvard University, that identified specific genetic markers connected to schizophrenia.
Watch It
Watch this video to learn more about the biological explanations of schizophrenia.
You can view the transcript for “Biological basis of schizophrenia | Behavior | MCAT | Khan Academy” here (opens in new window).
Try It
Glossary
dopamine-hypothesis: the oldest and most widely accepted biochemical model of schizophrenia that suggests overactivity of dopamine in the brain; newer studies are now exploring the role and interaction of other neurotransmitter systems
ventricles: hollow spaces in the brain
Candela Citations
- Twins. Authored by: LaWr123. Located at: https://commons.wikimedia.org/wiki/File:The_McClure_Twins.jpg. License: CC BY-SA: Attribution-ShareAlike
- Schizophrenia. Authored by: OpenStax College. Located at: http://cnx.org/contents/Sr8Ev5Og@5.52:gGD_wNTe@5/Schizophrenia. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/content/col11629/latest/.
- Causes of Schizophrenia. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Causes_of_schizophrenia#:~:text=Individual%20twin%20studies%20and%20meta,genetic%20determination%20of%20individual%20risk)%2C. License: CC BY-SA: Attribution-ShareAlike
- Biological basis of schizophrenia. Provided by: Khan Academy. Located at: https://www.youtube.com/watch?time_continue=1&v=6D_yOm6bjkw&feature=emb_logo. License: Other. License Terms: Standard YouTube License
- Piecing Together the Genetic Puzzle of Schizophrenia. Authored by: Joshua A. Gordon. Provided by: NIMH. Located at: https://www.nimh.nih.gov/about/director/messages/2020/piecing-together-the-genetic-puzzle-of-schizophrenia.shtml. License: Public Domain: No Known Copyright
- Siegmann A. E. (1976). A classification of sociomedical health indicators: perspectives for health administrators and health planners. International journal of health services : planning, administration, evaluation, 6(3), 521–538. https://doi.org/10.2190/MY7U-4BGM-9QFY-N0TN ↵