Learning Outcomes
- Define the octet rule and its role in chemical bonds
Not all elements have enough electrons to fill their outermost shells, but an atom is at its most stable when all of the electron positions in the outermost shell are filled. Because of these vacancies in the outermost shells, we see the formation of chemical bonds, or interactions between two or more of the same or different elements that result in the formation of molecules. To achieve greater stability, atoms will tend to completely fill their outer shells and will bond with other elements to accomplish this goal by sharing electrons, accepting electrons from another atom, or donating electrons to another atom. Because the outermost shells of the elements with low atomic numbers (up to calcium, with atomic number 20) can hold eight electrons, this is referred to as the octet rule. An element can donate, accept, or share electrons with other elements to fill its outer shell and satisfy the octet rule.
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885–1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. By convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between shells, an electron must absorb or release an amount of energy corresponding exactly to the difference in energy between the shells. For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat.
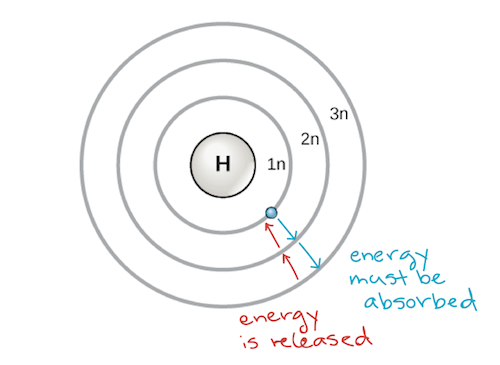
Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus. Energy must be added to move an electron outward to a higher energy level, and energy is released when an electron falls down from a higher energy level to a closer-in one. Image credit: modified from OpenStax Biology
Atoms, like other things governed by the laws of physics, tend to take on the lowest-energy, most stable configuration they can. Thus, the electron shells of an atom are populated from the inside out, with electrons filling up the low-energy shells closer to the nucleus before they move into the higher-energy shells further out. The shell closest to the nucleus, 1n, can hold two electrons, while the next shell, 2n, can hold eight, and the third shell, 3n, can hold up to eighteen.
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons. In general, atoms are most stable, least reactive, when their outermost electron shell is full. Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule. Some atoms can be stable with an octet even though their valence shell is the 3n shell, which can hold up to 18 electrons. We will explore the reason for this when we discuss electron orbitals below.
Examples of some neutral atoms and their electron configurations are shown below. In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Similarly, neon has a complete outer 2n shell containing eight electrons. These electron configurations make helium and neon very stable. Although argon does not technically have a full outer shell, since the 3n shell can hold up to eighteen electrons, it is stable like neon and helium because it has eight electrons in the 3n shell and thus satisfies the octet rule. In contrast, chlorine has only seven electrons in its outermost shell, while sodium has just one. These patterns do not fill the outermost shell or satisfy the octet rule, making chlorine and sodium reactive, eager to gain or lose electrons to reach a more stable configuration.
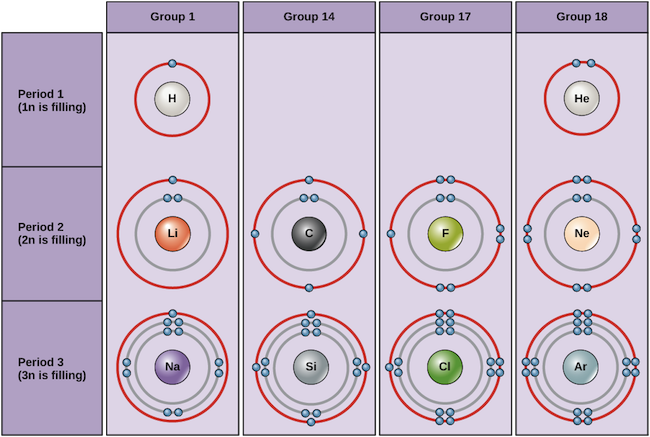
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration.
Electron configurations and the periodic table
Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number. In addition, the position of an element in the periodic table—its column, or group, and row, or period—provides useful information about how those electrons are arranged.
If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Similarly, an element’s column number gives information about its number of valence electrons and reactivity. In general, the number of valence electrons is the same within a column and increases from left to right within a row. Group 1 elements have just one valence electron and group 18 elements have eight, except for helium, which has only two electrons total. Thus, group number is a good predictor of how reactive each element will be:
- Helium (He), neon (Ne), and argon (Ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. This makes them highly stable as single atoms. Because of their non-reactivity, they are called the inert gases or noble gases.
- Hydrogen (H), lithium (Li), and sodium (Na), as group 1 elements, have just one electron in their outermost shells. They are unstable as single atoms, but can become stable by losing or sharing their one valence electron. If these elements fully lose an electron—as Li and Na typically do—they become positively charged ions: Li+, Na+.
- Fluorine (F) and chlorine (Cl), as group 17 elements, have seven electrons in their outermost shells. They tend to achieve a stable octet by taking an electron from other atoms, becoming negatively charged ions: F− and Cl−.
- Carbon (C), as a group 14 element, has four electrons in its outer shell. Carbon typically shares electrons to achieve a complete valence shell, forming bonds with multiple other atoms.
Thus, the columns of the periodic table reflect the number of electrons found in each element’s valence shell, which in turn determines how the element will react.
Try It
Candela Citations
- Concepts of Biology. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.25. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.25
- The periodic table, electron shells, and orbitals. Provided by: Khan Academy. Located at: https://www.khanacademy.org/science/biology/chemistry--of-life/electron-shells-and-orbitals/a/the-periodic-table-electron-shells-and-orbitals-article. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
