Learning Outcomes
- Model a hydrogen bond and identify its unique qualities
- Model van der Waals interactions and identify their unique qualities
Hydrogen Bonds
Ionic and covalent bonds between elements require energy to break. Ionic bonds are not as strong as covalent, which determines their behavior in biological systems. However, not all bonds are ionic or covalent bonds. Weaker bonds can also form between molecules. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist. Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and DNA, the building block of cells.
When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen’s electron is pulled more strongly toward the other element and away from the hydrogen. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When this happens, a weak interaction occurs between the δ+ of the hydrogen from one molecule and the δ– charge on the more electronegative atoms of another molecule, usually oxygen or nitrogen, or within the same molecule.
Hydrogen Bonding between water molecules
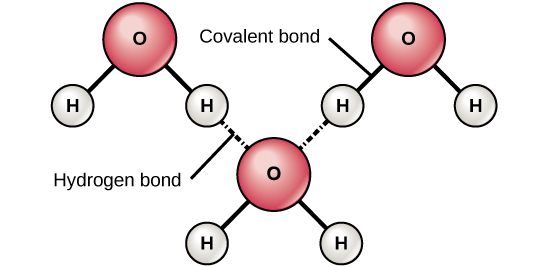
Figure 1. Hydrogen bonds form between slightly positive (δ+) and slightly negative (δ–) charges of polar covalent molecules, such as water.
This interaction is called a hydrogen bond. This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. Hydrogen bonds are also responsible for zipping together the DNA double helix.
Video Review
van der Waals Interactions
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. They are also called inter-molecular forces. They occur between polar, covalently bound atoms in different molecules. Some of these weak attractions are caused by temporary partial charges formed when electrons move around a nucleus. These weak interactions between molecules are important in biological systems and occur based on physical proximity.
Radiology Technician
Figure 2. Spc. Arbor L. LaClave practices his spinal X-ray positions utilizing Spc. Justin J. Reichelt, a radiology technician, as his mock patient to practice his skills in the health clinic at Grafenwoehr Training Area.
Have you or anyone you know ever had a magnetic resonance imaging (MRI) scan, a mammogram, or an X-ray? These tests produce images of your soft tissues and organs (as with an MRI or mammogram) or your bones (as happens in an X-ray) by using either radiowaves or special isotopes (radiolabeled or fluorescently labeled) that are ingested or injected into the body. These tests provide data for disease diagnoses by creating images of your organs or skeletal system.
MRI imaging works by subjecting hydrogen nuclei, which are abundant in the water in soft tissues, to fluctuating magnetic fields, which cause them to emit their own magnetic field. This signal is then read by sensors in the machine and interpreted by a computer to form a detailed image.
Some radiography technologists and technicians specialize in computed tomography, MRI, and mammography. They produce films or images of the body that help medical professionals examine and diagnose. Radiologists work directly with patients, explaining machinery, preparing them for exams, and ensuring that their body or body parts are positioned correctly to produce the needed images. Physicians or radiologists then analyze the test results.
Radiography technicians can work in hospitals, doctors’ offices, or specialized imaging centers. Training to become a radiography technician happens at hospitals, colleges, and universities that offer certificates, associate’s degrees, or bachelor’s degrees in radiography.
Try It
Try It
