Scientists use the term bioenergetics to describe the concept of energy flow (Figure 1) through living systems, such as cells. Cellular processes such as the building and breaking down of complex molecules occur through stepwise chemical reactions. Some of these chemical reactions are spontaneous and release energy, whereas others require energy to proceed.

Figure 1. Ultimately, most life forms get their energy from the sun. Plants use photosynthesis to capture sunlight, and herbivores eat the plants to obtain energy. Carnivores eat the herbivores, and eventual decomposition of plant and animal material contributes to the nutrient pool.
Just as living things must continually consume food to replenish their energy supplies, cells must continually produce more energy to replenish that used by the many energy-requiring chemical reactions that constantly take place. Together, all of the chemical reactions that take place inside cells, including those that consume or generate energy, are referred to as the cell’s metabolism.
Metabolic Pathways
Consider the metabolism of sugar. This is a classic example of one of the many cellular processes that use and produce energy. Living things consume sugars as a major energy source, because sugar molecules have a great deal of energy stored within their bonds. For the most part, photosynthesizing organisms like plants produce these sugars. During photosynthesis, plants use energy (originally from sunlight) to convert carbon dioxide gas (CO2) into sugar molecules (like glucose: C6H12O6). They consume carbon dioxide and produce oxygen as a waste product. This reaction is summarized as:
6CO2+6H2O→C6H12O6+6O2
Because this process involves synthesizing an energy-storing molecule, it requires energy input to proceed. During the light reactions of photosynthesis, energy is provided by a molecule called adenosine triphosphate (ATP), which is the primary energy currency of all cells. Just as the dollar is used as currency to buy goods, cells use molecules of ATP as energy currency to perform immediate work. In contrast, energy-storage molecules such as glucose are consumed only to be broken down to use their energy. The reaction that harvests the energy of a sugar molecule in cells requiring oxygen to survive can be summarized by the reverse reaction to photosynthesis. In this reaction, oxygen is consumed and carbon dioxide is released as a waste product. The reaction is summarized as:
C6H12O6→6H2O+6CO2
Both of these reactions involve many steps.
The processes of making and breaking down sugar molecules illustrate two examples of metabolic pathways. A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product. In the example of sugar metabolism, the first metabolic pathway synthesized sugar from smaller molecules, and the other pathway broke sugar down into smaller molecules. These two opposite processes—the first requiring energy and the second producing energy—are referred to as anabolic pathways (building polymers) and catabolic pathways (breaking down polymers into their monomers), respectively. Consequently, metabolism is composed of synthesis (anabolism) and degradation (catabolism) (Figure 2).

Figure 2. Catabolic pathways are those that generate energy by breaking down larger molecules. Anabolic pathways are those that require energy to synthesize larger molecules. Both types of pathways are required for maintaining the cell’s energy balance.
It is important to know that the chemical reactions of metabolic pathways do not take place on their own. Each reaction step is facilitated, or catalyzed, by a protein called an enzyme. Enzymes are important for catalyzing all types of biological reactions—those that require energy as well as those that release energy.
ATP In Living Systems
All living things require energy to function. While different organisms acquire this energy in different ways, they store (and use it) in the same way. In this section, we’ll learn about ATP—the energy of life. ATP is how cells store energy. These storage molecules are produced in the mitochondria, tiny organelles found in eukaryotic cells sometimes called the “powerhouse” of the cell.
MITOCHONDRIAL DISEASE PHYSICIAN
What happens when the critical reactions of cellular respiration do not proceed correctly? Mitochondrial diseases are genetic disorders of metabolism. Mitochondrial disorders can arise from mutations in nuclear or mitochondrial DNA, and they result in the production of less energy than is normal in body cells.
In type 2 diabetes, for instance, the oxidation efficiency of NADH is reduced, impacting oxidative phosphorylation but not the other steps of respiration. Symptoms of mitochondrial diseases can include muscle weakness, lack of coordination, stroke-like episodes, and loss of vision and hearing. Most affected people are diagnosed in childhood, although there are some adult-onset diseases.
Identifying and treating mitochondrial disorders is a specialized medical field. The educational preparation for this profession requires a college education, followed by medical school with a specialization in medical genetics. Medical geneticists can be board certified by the American Board of Medical Genetics and go on to become associated with professional organizations devoted to the study of mitochondrial diseases, such as the Mitochondrial Medicine Society and the Society for Inherited Metabolic Disease.
A living cell cannot store significant amounts of free energy. Excess free energy would result in an increase of heat in the cell, which would result in excessive thermal motion that could damage and then destroy the cell. Rather, a cell must be able to handle that energy in a way that enables the cell to store energy safely and release it for use only as needed. Living cells accomplish this by using the compound adenosine triphosphate (ATP). ATP is often called the “energy currency” of the cell, and, like currency, this versatile compound can be used to fill any energy need of the cell. How? It functions similarly to a rechargeable battery.
When ATP is broken down, usually by the removal of its terminal phosphate group, energy is released. The energy is used to do work by the cell, usually by the released phosphate binding to another molecule, activating it. For example, in the mechanical work of muscle contraction, ATP supplies the energy to move the contractile muscle proteins. Recall the active transport work of the sodium-potassium pump in cell membranes. ATP alters the structure of the integral protein that functions as the pump, changing its affinity for sodium and potassium. In this way, the cell performs work, pumping ions against their electrochemical gradients.
ATP Structure and Function
At the heart of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to a ribose molecule and to a single phosphate group (Figure 3). Ribose is a five-carbon sugar found in RNA, and AMP is one of the nucleotides in RNA. The addition of a second phosphate group to this core molecule results in the formation of adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).

Figure 3. ATP (adenosine triphosphate) has three phosphate groups that can be removed by hydrolysis to form ADP (adenosine diphosphate) or AMP (adenosine monophosphate).The negative charges on the phosphate group naturally repel each other, requiring energy to bond them together and releasing energy when these bonds are broken.
The addition of a phosphate group to a molecule requires energy. Phosphate groups are negatively charged and thus repel one another when they are arranged in series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. The release of one or two phosphate groups from ATP, a process called dephosphorylation, releases energy.
Energy from ATP
Hydrolysis is the process of breaking complex macromolecules apart. During hydrolysis, water is split, or lysed, and the resulting hydrogen atom (H+) and a hydroxyl group (OH–) are added to the larger molecule. The hydrolysis of ATP produces ADP, together with an inorganic phosphate ion (Pi), and the release of free energy. To carry out life processes, ATP is continuously broken down into ADP, and like a rechargeable battery, ADP is continuously regenerated into ATP by the reattachment of a third phosphate group. Water, which was broken down into its hydrogen atom and hydroxyl group during ATP hydrolysis, is regenerated when a third phosphate is added to the ADP molecule, reforming ATP.
Obviously, energy must be infused into the system to regenerate ATP. Where does this energy come from? In nearly every living thing on earth, the energy comes from the metabolism of glucose. In this way, ATP is a direct link between the limited set of exergonic pathways of glucose catabolism and the multitude of endergonic pathways that power living cells.
Phosphorylation
Recall that, in some chemical reactions, enzymes may bind to several substrates that react with each other on the enzyme, forming an intermediate complex. An intermediate complex is a temporary structure, and it allows one of the substrates (such as ATP) and reactants to more readily react with each other; in reactions involving ATP, ATP is one of the substrates and ADP is a product. During an endergonic chemical reaction, ATP forms an intermediate complex with the substrate and enzyme in the reaction. This intermediate complex allows the ATP to transfer its third phosphate group, with its energy, to the substrate, a process called phosphorylation.
When the intermediate complex breaks apart, the energy is used to modify the substrate and convert it into a product of the reaction. The ADP molecule and a free phosphate ion are released into the medium and are available for recycling through cell metabolism.

Virtually every task performed by living organisms requires energy. Energy is needed to perform heavy labor and exercise, but humans also use energy while thinking, and even during sleep. In fact, the living cells of every organism constantly use energy.
Just as energy is required to both build and demolish a building, energy is required for the synthesis and breakdown of molecules as well as the transport of molecules into and out of cells. In addition, processes such as ingesting and breaking down pathogenic bacteria and viruses, exporting wastes and toxins, and movement of the cell require energy. Nutrients and other molecules are imported into the cell, metabolized (broken down) and possibly synthesized into new molecules, modified if needed, transported around the cell, and possibly distributed to the entire organism. For example, the large proteins that make up muscles are built from smaller molecules imported from dietary amino acids. Complex carbohydrates are broken down into simple sugars that the cell uses for energy.
Cellular Respiration: Synthesis of ATP
Cellular respiration extracts the energy from the bonds in glucose and converts it into a form that all living things can use. Now let’s take a more detailed look at how all eukaryotes—which includes humans!—make use of this stored energy. There are two types of cellular respiration: 1. aerobic and 2. anaerobic. Aerobic respiration requires oxygen and yields high amounts of ATP per one glucose molecule compared to anaerobic that does not require oxygen and yields low levels of ATP per glucose molecule. Both aerobic and anaerobic respiration begin with process called glycolysis.
Glycolysis
You have read that nearly all of the energy used by living things comes to them in the bonds of the sugar, glucose. Glycolysis is the first step in the breakdown of glucose to extract energy for cell metabolism. Many living organisms carry out glycolysis as part of their metabolism. Glycolysis takes place in the cytoplasm of most prokaryotic and all eukaryotic cells.
Glycolysis begins with the six-carbon, ring-shaped structure of a single glucose molecule and ends with two molecules of a three-carbon sugar called pyruvate. Glycolysis consists of two distinct phases. In the first part of the glycolysis pathway, energy is used to make adjustments so that the six-carbon sugar molecule can be split evenly into two three-carbon pyruvate molecules. In the second part of glycolysis, ATP and nicotinamide-adenine dinucleotide (NADH) are produced (Figure 5).

Figure 5. In glycolysis, a glucose molecule is converted into two pyruvate molecules.
If the cell cannot catabolize the pyruvate molecules further, it will harvest only two ATP molecules from one molecule of glucose. For example, mature mammalian red blood cells are only capable of glycolysis, which is their sole source of ATP. If glycolysis is interrupted, these cells would eventually die.
Aerobic Respiration: Citric Acid Cycle and Oxidative Phosphorylation
In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are sites of cellular respiration. If oxygen is available, aerobic respiration will go forward. There are two main processes of aerobic respiration: The Citric Acid Cycle (or the Kreb’s Cycle) and Oxidative Phosphorylation.
The Citric Acid Cycle
In mitochondria, pyruvate will be transformed into a two-carbon acetyl group (by removing a molecule of carbon dioxide) that will be picked up by a carrier compound called coenzyme A (CoA), which is made from vitamin B5. The resulting compound is called acetyl CoA. (Figure 6). Acetyl CoA can be used in a variety of ways by the cell, but its major function is to deliver the acetyl group derived from pyruvate to the next pathway in glucose catabolism.

Figure 6. Pyruvate is converted into acetyl-CoA before entering the citric acid cycle.
Like the conversion of pyruvate to acetyl CoA, the citric acid cycle in eukaryotic cells takes place in the matrix of the mitochondria. Unlike glycolysis, the citric acid cycle is a closed loop: The last part of the pathway regenerates the compound used in the first step. The eight steps of the cycle are a series of chemical reactions that produces two carbon dioxide molecules, one ATP molecule (or an equivalent), and reduced forms (NADH and FADH2) of NAD+ and FAD+, important coenzymes in the cell. Part of this is considered an aerobic pathway (oxygen-requiring) because the NADH and FADH2 produced must transfer their electrons to the next pathway in the system, which will use oxygen. If oxygen is not present, this transfer does not occur.
Two carbon atoms come into the citric acid cycle from each acetyl group. Two carbon dioxide molecules are released on each turn of the cycle; however, these do not contain the same carbon atoms contributed by the acetyl group on that turn of the pathway. The two acetyl-carbon atoms will eventually be released on later turns of the cycle; in this way, all six carbon atoms from the original glucose molecule will be eventually released as carbon dioxide. It takes two turns of the cycle to process the equivalent of one glucose molecule. Each turn of the cycle forms three high-energy NADH molecules and one high-energy FADH2 molecule. These high-energy carriers will connect with the last portion of aerobic respiration to produce ATP molecules. One ATP (or an equivalent) is also made in each cycle. Several of the intermediate compounds in the citric acid cycle can be used in synthesizing non-essential amino acids; therefore, the cycle is both anabolic and catabolic.
Oxidative Phosphorylation
You have just read about two pathways in glucose catabolism—glycolysis and the citric acid cycle—that generate ATP. Most of the ATP generated during the aerobic catabolism of glucose, however, is not generated directly from these pathways. Rather, it derives from a process that begins with passing electrons through a series of chemical reactions to a final electron acceptor, oxygen. These reactions take place in specialized protein complexes located in the inner membrane of the mitochondria of eukaryotic organisms and on the inner part of the cell membrane of prokaryotic organisms. The energy of the electrons is harvested and used to generate a electrochemical gradient across the inner mitochondrial membrane. The potential energy of this gradient is used to generate ATP. The entirety of this process is called oxidative phosphorylation.
The electron transport chain (Figure 7a) is the last component of aerobic respiration and is the only part of metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants for this purpose. In animals, oxygen enters the body through the respiratory system. Electron transport is a series of chemical reactions that resembles a bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain where oxygen is the final electron acceptor and water is produced.
The electron transport chain are four complexes composed of proteins and accessory electron carriers. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes. In each transfer of an electron through the electron transport chain, the electron loses energy, but with some transfers, the energy is stored as potential energy by using it to pump hydrogen ions across the inner mitochondrial membrane into the intermembrane space, creating an electrochemical gradient. This process is called chemiosmosis (7c).

Figure 7. (a) The electron transport chain is a set of molecules that supports a series of oxidation-reduction reactions. (b) ATP synthase is a complex, molecular machine that uses an H+ gradient to regenerate ATP from ADP. (c) Chemiosmosis relies on the potential energy provided by the H+ gradient across the membrane.
Chemiosmosis
Chemiosmosis is the movement of ions across a semipermeable membrane, down their electrochemical gradient. Hydrogen ions, or protons, will diffuse from an area of high proton concentration to an area of lower proton concentration. This electrochemical concentration gradient of protons can be used to make ATP.
ATP synthase is the enzyme that makes ATP by chemiosmosis. It allows protons to pass through the membrane and uses the free energy difference to phosphorylate adenosine diphosphate (ADP), making ATP. The energy from the electron movement through electron transport chains to ATP synthase allows the proton to pass through ATP synthase to photophosphorylate ADP making ATP.
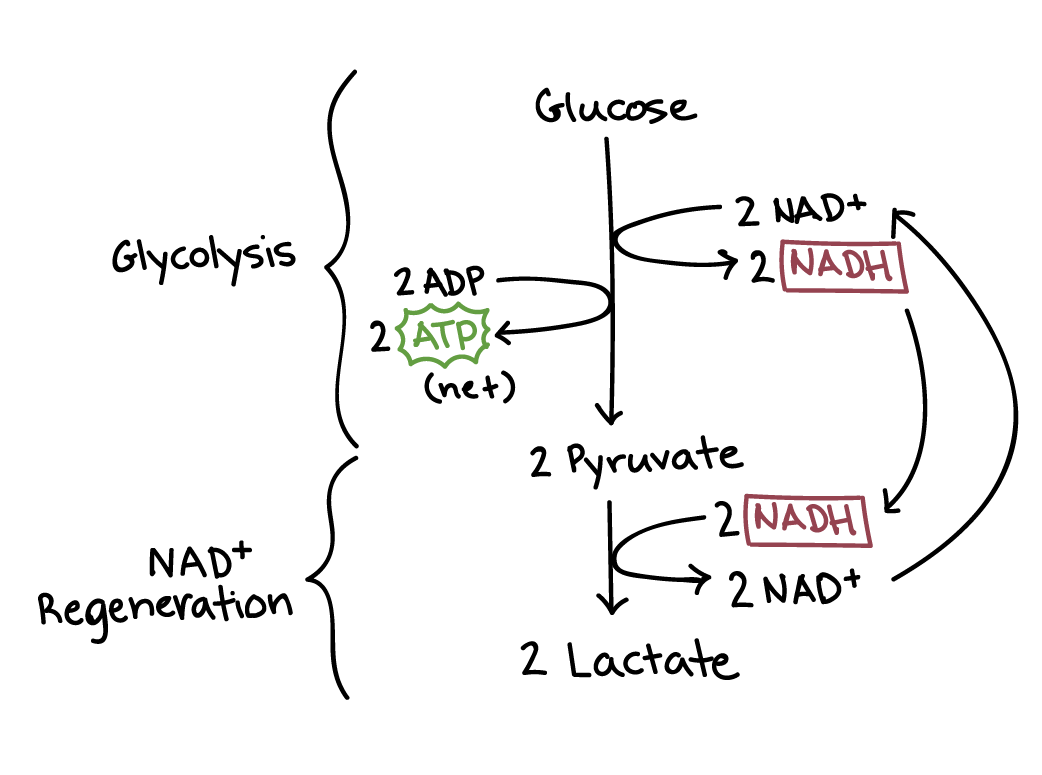 Lactic acid fermentation has two steps: glycolysis and NADH regeneration.
Lactic acid fermentation has two steps: glycolysis and NADH regeneration.