Learning Objectives
- Relate the structure of the heart to its function as a pump
- Identify and describe the interior and exterior parts of the human heart
- Compare cardiac muscle to skeletal and smooth muscle
- Explain the cardiac cycle
- Compare systemic circulation to pulmonary circulation
- Identify and describe the components of the conducting system that distributes electrical impulses through the heart
- Describe factors affecting heart rate
- Compare and contrast the anatomical structure of arteries, arterioles, capillaries, venules, and veins
The vital importance of the heart is obvious. If one assumes an average rate of 75 contractions per minute, a human heart would contract approximately 108,000 times in one day, more than 39 million times in one year, and nearly 3 billion times during a 75-year lifespan. Each of the major pumping chambers of the heart ejects approximately 70 mL blood per contraction in a resting adult. This would be equal to 5.25 liters of fluid per minute and approximately 14,000 liters per day. Over one year, that would equal 10,000,000 liters or 2.6 million gallons of blood sent through roughly 60,000 miles of vessels. In order to understand how that happens, it is necessary to understand the anatomy and physiology of the heart.
Location of the Heart
The human heart is located within the thoracic cavity, between the lungs in the space known as the mediastinum. Figure 1 shows the position of the heart within the thoracic cavity. Within the mediastinum, the heart is enclosed by a tough membrane known as the pericardium, or pericardial sac, and sits in its own space called the pericardial cavity. The dorsal surface of the heart lies near the bodies of the vertebrae, and its anterior surface sits deep to the sternum and costal cartilages. The great veins, the superior and inferior venae cavae, and the great arteries, the aorta and pulmonary trunk, are attached to the superior surface of the heart, called the base. The inferior tip of the heart, the apex, lies just to the left of the sternum.

Figure 1. The heart is located within the thoracic cavity, medially between the lungs in the mediastinum. It is about the size of a fist, is broad at the top, and tapers toward the base.
Everyday Connection: CPR
The position of the heart in the torso between the vertebrae and sternum (see the image above for the position of the heart within the thorax) allows for individuals to apply an emergency technique known as cardiopulmonary resuscitation (CPR) if the heart of a patient should stop. By applying pressure with the flat portion of one hand on the sternum in the area between the lines in the image below), it is possible to manually compress the blood within the heart enough to push some of the blood within it into the pulmonary and systemic circuits. This is particularly critical for the brain, as irreversible damage and death of neurons occur within minutes of loss of blood flow.
Figure 2. If the heart should stop, CPR can maintain the flow of blood until the heart resumes beating. By applying pressure to the sternum, the blood within the heart will be squeezed out of the heart and into the circulation. Proper positioning of the hands on the sternum to perform CPR would be between the lines at T4 and T9.
Shape and Size of the Heart
The shape of the heart is similar to a pinecone, rather broad at the superior surface and tapering to the apex. A typical heart is approximately the size of your fist: 12 cm (5 in) in length, 8 cm (3.5 in) wide, and 6 cm (2.5 in) in thickness. The heart of a well-trained athlete, especially one specializing in aerobic sports, can be considerably larger than this. Cardiac muscle responds to exercise in a manner similar to that of skeletal muscle. That is, exercise results in the addition of protein myofilaments that increase the size of the individual cells without increasing their numbers, a concept called hypertrophy. As a result of this hypertrophy athletic hearts can pump blood more effectively at lower rates than nonathletic hearts.
Chambers and Circulation through the Heart
The human heart consists of four chambers: The left side and the right side each have one atrium and one ventricle. Each of the upper chambers, the right atrium (plural = atria) and the left atrium, acts as a receiving chamber and contracts to push blood into the lower chambers, the right ventricle and the left ventricle. The ventricles serve as the primary pumping chambers of the heart, propelling blood to the lungs or to the rest of the body.
There are two distinct but linked circuits in the human circulation called the pulmonary and systemic circuits. Although both circuits transport blood and everything it carries, we can initially view the circuits from the point of view of gases. The pulmonary circuit transports blood to and from the lungs, where it picks up oxygen and delivers carbon dioxide for exhalation. The systemic circuit transports oxygenated blood to virtually all of the tissues of the body and returns relatively deoxygenated blood and carbon dioxide to the heart to be sent back to the pulmonary circulation.
The right ventricle pumps deoxygenated blood into the pulmonary trunk, which leads toward the lungs and branches into the left and right pulmonary arteries. These vessels in turn branch many times before reaching the pulmonary capillaries, where gas exchange occurs: Carbon dioxide exits the blood and oxygen enters. The pulmonary trunk arteries and their branches are the only arteries in the post-natal body that carry relatively deoxygenated blood. Highly oxygenated blood returning from the pulmonary capillaries in the lungs passes through a series of vessels that join together to form the pulmonary veins—the only post-natal veins in the body that carry highly oxygenated blood. The pulmonary veins direct blood into the left atrium, which pumps the blood into the left ventricle, which in turn pumps oxygenated blood into the aorta and on to the many branches of the systemic circuit. Eventually, these vessels will lead to the systemic capillaries, where exchange with the tissue fluid and cells of the body occurs. In this case, oxygen and nutrients exit the systemic capillaries to be used by the cells in their metabolic processes, and carbon dioxide and waste products will enter the blood.
The blood exiting the systemic capillaries is lower in oxygen concentration than when it entered. The capillaries will ultimately unite to form venules, joining to form ever-larger veins, eventually flowing into the two major systemic veins, the superior vena cava and the inferior vena cava, which return blood to the right atrium. The blood in the superior and inferior venae cavae flows into the right atrium, which pumps blood into the right ventricle. This process of blood circulation continues as long as the individual remains alive.

Figure 3. Blood flows from the right atrium to the right ventricle, where it is pumped into the pulmonary circuit. The blood in the pulmonary artery branches is low in oxygen but relatively high in carbon dioxide. Gas exchange occurs in the pulmonary capillaries (oxygen into the blood, carbon dioxide out), and blood high in oxygen and low in carbon dioxide is returned to the left atrium. From here, blood enters the left ventricle, which pumps it into the systemic circuit. Following exchange in the systemic capillaries (oxygen and nutrients out of the capillaries and carbon dioxide and wastes in), blood returns to the right atrium and the cycle is repeated.
Membranes

Figure 4. The pericardial membrane that surrounds the heart consists of three layers and the pericardial cavity. The heart wall also consists of three layers. The pericardial membrane and the heart wall share the epicardium.
The membrane that directly surrounds the heart and defines the pericardial cavity is called the pericardium or pericardial sac. The pericardium, which literally translates as “around the heart,” consists of two distinct sublayers: the sturdy outer fibrous pericardium and the inner serous pericardium. The fibrous pericardium is made of tough, dense connective tissue that protects the heart and maintains its position in the thorax. The more delicate serous pericardium consists of two layers: the parietal pericardium, which is fused to the fibrous pericardium, and an inner visceral pericardium, or epicardium, which is fused to the heart and is part of the heart wall. The pericardial cavity, filled with lubricating serous fluid, lies between the epicardium and the pericardium.
Disorders of the Heart
Cardiac Tamponade
If excess fluid builds within the pericardial space, it can lead to a condition called cardiac tamponade, or pericardial tamponade. With each contraction of the heart, more fluid—in most instances, blood—accumulates within the pericardial cavity. In order to fill with blood for the next contraction, the heart must relax. However, the excess fluid in the pericardial cavity puts pressure on the heart and prevents full relaxation, so the chambers within the heart contain slightly less blood as they begin each heart cycle. Over time, less and less blood is ejected from the heart. If the fluid builds up slowly, as in hypothyroidism, the pericardial cavity may be able to expand gradually to accommodate this extra volume. Some cases of fluid in excess of one liter within the pericardial cavity have been reported. Rapid accumulation of as little as 100 mL of fluid following trauma may trigger cardiac tamponade. Other common causes include myocardial rupture, pericarditis, cancer, or even cardiac surgery. Removal of this excess fluid requires insertion of drainage tubes into the pericardial cavity. Premature removal of these drainage tubes, for example, following cardiac surgery, or clot formation within these tubes are causes of this condition. Untreated, cardiac tamponade can lead to death.
Layers
The wall of the heart is composed of three layers of unequal thickness. From superficial to deep, these are the epicardium, the myocardium, and the endocardium. The outermost layer of the wall of the heart is also the innermost layer of the pericardium, the epicardium, or the visceral pericardium discussed earlier.

Figure 6. The swirling pattern of cardiac muscle tissue contributes significantly to the heart’s ability to pump blood effectively.
The middle and thickest layer is the myocardium, which consists of cardiac muscle cells. It is the contraction of the myocardium that pumps blood through the heart and into the major arteries. The muscle pattern is elegant and complex, as the muscle cells swirl and spiral around the chambers of the heart. They form a figure 8 pattern around the atria and around the bases of the great vessels. Deeper ventricular muscles also form a figure 8 around the two ventricles and proceed toward the apex. This complex swirling pattern allows the heart to pump blood more effectively than a simple linear pattern would. Figure 6 illustrates the arrangement of muscle cells.
The muscle of the left ventricle is much thicker and better developed than that of the right ventricle. In order to overcome the high resistance required to pump blood into the long systemic circuit, the left ventricle must generate a great amount of pressure. The right ventricle does not need to generate as much pressure, since the pulmonary circuit is shorter and provides less resistance. The image below illustrates the differences in muscular thickness needed for each of the ventricles.

Figure 7. The myocardium in the left ventricle is significantly thicker than that of the right ventricle. The left ventricle must generate a much greater pressure to overcome greater resistance in the systemic circuit. The ventricles are shown in both relaxed and contracting states.
The innermost layer of the heart wall, the endocardium, is joined to the myocardium with a thin layer of connective tissue. The endocardium lines the chambers where the blood circulates and covers the heart valves. It is made of simple squamous epithelium called endothelium, which is continuous with the endothelial lining of the blood vessels.
Internal Structure of the Heart
Recall that the heart’s contraction cycle follows a dual pattern of circulation—the pulmonary and systemic circuits—because of the pairs of chambers that pump blood into the circulation. In order to develop a more precise understanding of cardiac function, it is first necessary to explore the internal anatomical structures in more detail.
Septa of the Heart
The word septum is derived from the Latin for “something that encloses;” in this case, a septum (plural = septa) refers to a wall or partition that divides the heart into chambers. The septa are physical extensions of the myocardium lined with endocardium. Located between the two atria is the interatrial septum. Between the two ventricles is a second septum known as the interventricular septum. It is substantially thicker than the interatrial septum, since the ventricles generate far greater pressure when they contract.
The septum between the atria and ventricles is known as the atrioventricular septum. It is marked by the presence of four openings that allow blood to move from the atria into the ventricles and from the ventricles into the pulmonary trunk and aorta. Located in each of these openings between the atria and ventricles is a valve, a specialized structure that ensures one-way flow of blood.
The valves between the atria and ventricles are known generically as atrioventricular valves. The valves at the openings that lead to the pulmonary trunk and aorta are known generically as semilunar valves. The interventricular septum is visible in the image below. In this figure, the atrioventricular septum has been removed to better show the bicuspid and tricuspid valves; the interatrial septum is not visible, since its location is covered by the aorta and pulmonary trunk.

Figure 8. This anterior view of the heart shows the four chambers, the major vessels and their early branches, as well as the valves. The presence of the pulmonary trunk and aorta covers the interatrial septum, and the atrioventricular septum is cut away to show the atrioventricular valves.
Disorders of the Heart: Heart Defects
One very common form of interatrial septum pathology is patent foramen ovale, which occurs when the septum does not close at birth, and an opening remains between the left and right atria. The word patent is from the Latin root patens for “open.” It may be benign or asymptomatic, perhaps never being diagnosed, or in extreme cases, it may require surgical repair to close the opening permanently. As much as 20–25 percent of the general population may have a patent foramen ovale, but fortunately, most have the benign, asymptomatic version. Patent foramen ovale is normally detected by presence of a heart murmur (an abnormal heart sound) and confirmed by imaging with an echocardiogram. Despite its prevalence in the general population, the causes of patent ovale are unknown, and there are no known risk factors. In nonlife-threatening cases, it is better to monitor the condition than to risk heart surgery to repair and seal the opening.

Figure 9. A patent foramen ovale defect is an abnormal opening in the interatrial septum, or more commonly, a failure of the foramen ovale to close.
Right Atrium
The right atrium serves as the receiving chamber for blood returning to the heart from the systemic circulation. The two major systemic veins, the superior and inferior venae cavae, and the large coronary vein called the coronary sinus that drains the heart myocardium empty into the right atrium. The superior vena cava drains blood from regions superior to the diaphragm: the head, neck, upper limbs, and the thoracic region. The inferior vena cava drains blood from areas inferior to the diaphragm: the lower limbs and abdominopelvic region of the body. The coronary sinus is a thin-walled vessel that drains most of the coronary veins that return systemic blood from the heart. The majority of the internal heart structures discussed in this and subsequent sections are illustrated in Figure 8.
Right Ventricle
The right ventricle receives blood from the right atrium through the tricuspid valve. Each flap of the valve is attached to strong strands of connective tissue, the chordae tendineae, literally “tendinous cords,” or sometimes more poetically referred to as “heart strings.” There are several chordae tendineae associated with each of the flaps. They connect each of the flaps to a papillary muscle that extends from the inferior ventricular surface.
When the myocardium of the ventricle contracts, pressure within the ventricular chamber rises. To prevent any potential backflow, the papillary muscles also contract, generating tension on the chordae tendineae. This prevents the flaps of the valves from being forced into the atria and regurgitation of the blood back into the atria during ventricular contraction.The image below shows papillary muscles and chordae tendineae attached to the tricuspid valve.

Figure 10. In this frontal section, you can see papillary muscles attached to the tricuspid valve on the right as well as the mitral valve on the left via chordae tendineae. (credit: modification of work by “PV KS”/flickr.com)
When the right ventricle contracts, it ejects blood into the pulmonary trunk, which branches into the left and right pulmonary arteries that carry it to each lung. The superior surface of the right ventricle begins to taper as it approaches the pulmonary trunk. At the base of the pulmonary trunk is the pulmonary semilunar valve that prevents backflow from the pulmonary trunk.
Left Atrium
After exchange of gases in the pulmonary capillaries, blood returns to the left atrium high in oxygen via one of the four pulmonary veins. Blood flows nearly continuously from the pulmonary veins back into the atrium, which acts as the receiving chamber, and from here through an opening into the left ventricle. Most blood flows passively into the heart while both the atria and ventricles are relaxed, but toward the end of the ventricular relaxation period, the left atrium will contract, pumping blood into the ventricle. This atrial contraction accounts for approximately 20 percent of ventricular filling. The opening between the left atrium and ventricle is guarded by the mitral valve.
Left Ventricle
Recall that the muscular layer is much thicker in the left ventricle compared to the right. The mitral valve is connected to papillary muscles via chordae tendineae. There are two papillary muscles on the left—the anterior and posterior—as opposed to three on the right.
The left ventricle is the major pumping chamber for the systemic circuit; it ejects blood into the aorta through the aortic semilunar valve.
Heart Valve Function
In Figure 12 (a), the two atrioventricular valves are open and the two semilunar valves are closed. This occurs when both atria and ventricles are relaxed and when the atria contract to pump blood into the ventricles. Figure 13 (b) shows a frontal view. Although only the left side of the heart is illustrated, the process is virtually identical on the right.

Figure 12. (a) A transverse section through the heart illustrates the four heart valves. The two atrioventricular valves are open; the two semilunar valves are closed. The atria and vessels have been removed. (b) A frontal section through the heart illustrates blood flow through the mitral valve. When the mitral valve is open, it allows blood to move from the left atrium to the left ventricle. The aortic semilunar valve is closed to prevent backflow of blood from the aorta to the left ventricle.
Figure 13 (a) shows the atrioventricular valves closed while the two semilunar valves are open. This occurs when the ventricles contract to eject blood into the pulmonary trunk and aorta. Closure of the two atrioventricular valves prevents blood from being forced back into the atria. This stage can be seen from a frontal view in Figure 13 (b).
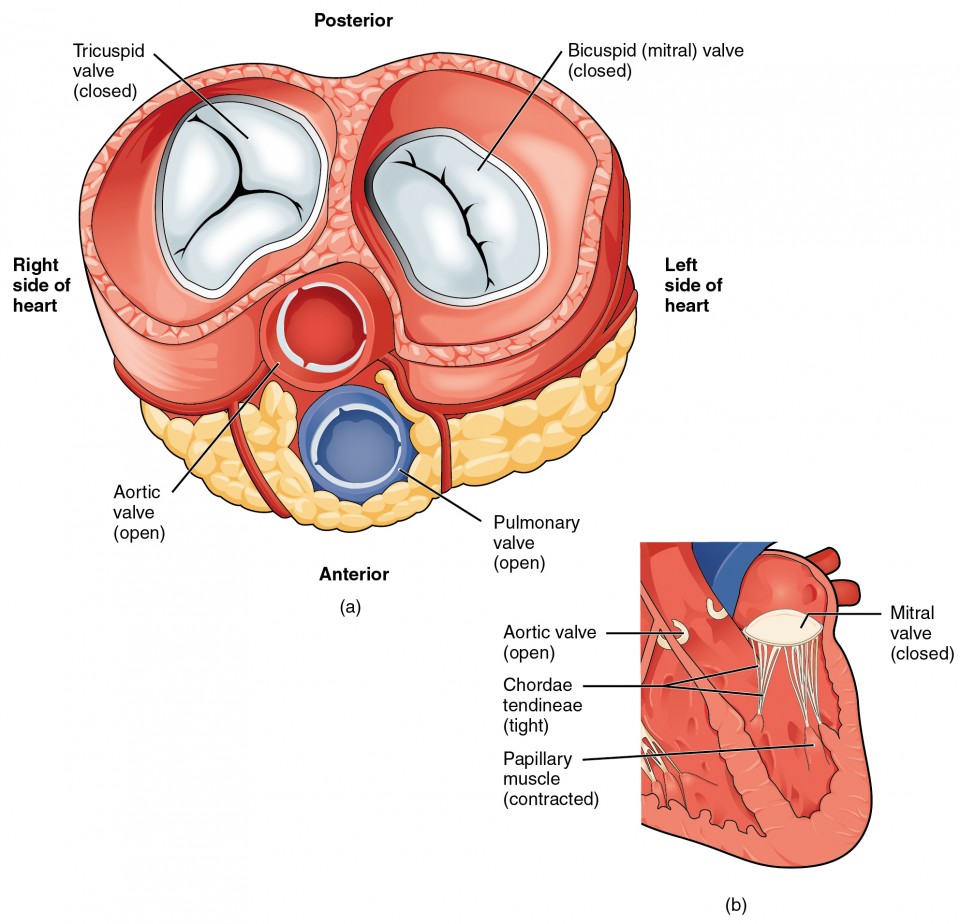
Figure 13. (a) A transverse section through the heart illustrates the four heart valves during ventricular contraction. The two atrioventricular valves are closed, but the two semilunar valves are open. The atria and vessels have been removed. (b) A frontal view shows the closed mitral (bicuspid) valve that prevents backflow of blood into the left atrium. The aortic semilunar valve is open to allow blood to be ejected into the aorta.
Figure 14 shows an echocardiogram of actual heart valves opening and closing. Although much of the heart has been “removed” from this gif loop so the chordae tendineae are not visible. The pressure gradient between the atria and the ventricles is much greater than that between the ventricles and the pulmonary trunk and aorta. Without the presence of the chordae tendineae and papillary muscles, the valves would be blown back (prolapsed) into the atria and blood would regurgitate.

Figure 14. An echocardiogram of heart valves
Disorders of the Heart Valves
When heart valves do not function properly, they are often described as incompetent and result in valvular heart disease, which can range from benign to lethal. Some of these conditions are congenital, that is, the individual was born with the defect, whereas others may be attributed to disease processes or trauma. Some malfunctions are treated with medications, others require surgery, and still others may be mild enough that the condition is merely monitored since treatment might trigger more serious consequences.
Valvular disorders are often caused by carditis, or inflammation of the heart. One common trigger for this inflammation is rheumatic fever, or scarlet fever, an autoimmune response to the presence of a bacterium, Streptococcus pyogenes, normally a disease of childhood.
While any of the heart valves may be involved in valve disorders, mitral regurgitation is the most common, detected in approximately 2 percent of the population, and the pulmonary semilunar valve is the least frequently involved. When a valve malfunctions, the flow of blood to a region will often be disrupted.
If one of the cusps of the valve is forced backward by the force of the blood, the condition is referred to as a prolapsed valve. Prolapse may occur if the chordae tendineae are damaged or broken, causing the closure mechanism to fail. The failure of the valve to close properly disrupts the normal one-way flow of blood and results in regurgitation, when the blood flows backward from its normal path. Using a stethoscope, the disruption to the normal flow of blood produces a heart murmur.
Auscultation, or listening to a patient’s heart sounds, is one of the most useful diagnostic tools, since it is proven, safe, and inexpensive. The term auscultation is derived from the Latin for “to listen,” and the technique has been used for diagnostic purposes as far back as the ancient Egyptians. Valve and septal disorders will trigger abnormal heart sounds. If a valvular disorder is detected or suspected, a test called an echocardiogram, or simply an “echo,” may be ordered. Echocardiograms are sonograms of the heart and can help in the diagnosis of valve disorders as well as a wide variety of heart pathologies.
Coronary Circulation
You will recall that the heart is a remarkable pump composed largely of cardiac muscle cells that are incredibly active throughout life. Like all other cells, a cardiac muscle cell requires a reliable supply of oxygen and nutrients, and a way to remove wastes, so it needs a dedicated, complex, and extensive coronary circulation. And because of the critical and nearly ceaseless activity of the heart throughout life, this need for a blood supply is even greater than for a typical cell.
Coronary Arteries
Coronary arteries supply blood to the myocardium and other components of the heart. The first portion of the aorta after it arises from the left ventricle gives rise to the coronary arteries. The left coronary artery distributes blood to the left side of the heart, the left atrium and ventricle, and the interventricular septum. The right coronary artery distributes blood to the right atrium, portions of both ventricles, and the heart conduction system. Figure 15 presents views of the coronary circulation from both the anterior and posterior views.
Coronary Veins
Coronary veins drain the heart and generally parallel the large surface arteries. The coronary sinus is a large, thin-walled vein on the posterior surface of the heart that empties directly into the right atrium. The anterior cardiac veins parallel the small cardiac arteries and drain the anterior surface of the right ventricle. Unlike other cardiac veins, it bypasses the coronary sinus and drains directly into the right atrium.

Figure 15. The anterior view of the heart shows the prominent coronary surface vessels. The posterior view of the heart shows the prominent coronary surface vessels.
Diseases of the Heart: Myocardial Infarction
Myocardial infarction (MI) is the formal term for what is commonly referred to as a heart attack. It normally results from a lack of blood flow (ischemia) and oxygen (hypoxia) to a region of the heart, resulting in death of the cardiac muscle cells. A myocardial infarction often occurs when a coronary artery is blocked by the buildup of atherosclerotic plaque consisting of lipids, cholesterol and fatty acids, and white blood cells, primarily macrophages. It can also occur when a portion of an unstable atherosclerotic plaque travels through the coronary arterial system and lodges in one of the smaller vessels. The resulting blockage restricts the flow of blood and oxygen to the myocardium and causes death of the tissue. MIs may be triggered by excessive exercise, in which the partially occluded artery is no longer able to pump sufficient quantities of blood, or severe stress, which may induce spasm of the smooth muscle in the walls of the vessel.
In the case of acute MI, there is often sudden pain beneath the sternum (retrosternal pain) called angina pectoris, often radiating down the left arm in males but not in female patients. Until this anomaly between the sexes was discovered, many female patients suffering MIs were misdiagnosed and sent home. In addition, patients typically present with difficulty breathing and shortness of breath (dyspnea), irregular heartbeat (palpations), nausea and vomiting, sweating (diaphoresis), anxiety, and fainting (syncope), although not all of these symptoms may be present. Many of the symptoms are shared with other medical conditions, including anxiety attacks and simple indigestion, so differential diagnosis is critical. It is estimated that between 22 and 64 percent of MIs present without any symptoms.
An MI can be confirmed by examining the patient’s ECG, which frequently reveals alterations in the ST and Q components. Some classification schemes of MI are referred to as ST-elevated MI (STEMI) and non-elevated MI (non-STEMI). In addition, echocardiography or cardiac magnetic resonance imaging may be employed. Common blood tests indicating an MI include elevated levels of creatine kinase MB (an enzyme that catalyzes the conversion of creatine to phosphocreatine, consuming ATP) and cardiac troponin (the regulatory protein for muscle contraction), both of which are released by damaged cardiac muscle cells.
Immediate treatments for MI are essential and include administering supplemental oxygen, aspirin that helps to break up clots, and nitroglycerine administered sublingually (under the tongue) to facilitate its absorption. Despite its unquestioned success in treatments and use since the 1880s, the mechanism of nitroglycerine is still incompletely understood but is believed to involve the release of nitric oxide, a known vasodilator, and endothelium-derived releasing factor, which also relaxes the smooth muscle in the tunica media of coronary vessels. Longer-term treatments include injections of thrombolytic agents such as streptokinase that dissolve the clot, the anticoagulant heparin, balloon angioplasty and stents to open blocked vessels, and bypass surgery to allow blood to pass around the site of blockage. If the damage is extensive, coronary replacement with a donor heart or coronary assist device, a sophisticated mechanical device that supplements the pumping activity of the heart, may be employed. Despite the attention, development of artificial hearts to augment the severely limited supply of heart donors has proven less than satisfactory but will likely improve in the future.
MIs may trigger cardiac arrest, but the two are not synonymous. Important risk factors for MI include cardiovascular disease, age, smoking, high blood levels of the low-density lipoprotein (LDL, often referred to as “bad” cholesterol), low levels of high-density lipoprotein (HDL, or “good” cholesterol), hypertension, diabetes mellitus, obesity, lack of physical exercise, chronic kidney disease, excessive alcohol consumption, and use of illegal drugs.
Diseases of the heart: coronary artery disease
Coronary artery disease is the leading cause of death worldwide. It occurs when the buildup of plaque—a fatty material including cholesterol, connective tissue, white blood cells, and some smooth muscle cells—within the walls of the arteries obstructs the flow of blood and decreases the flexibility or compliance of the vessels. This condition is called atherosclerosis, a hardening of the arteries that involves the accumulation of plaque. As the coronary blood vessels become occluded, the flow of blood to the tissues will be restricted, a condition called ischemia that causes the cells to receive insufficient amounts of oxygen, called hypoxia. The image below shows the blockage of coronary arteries highlighted by the injection of dye. Some individuals with coronary artery disease report pain radiating from the chest called angina pectoris, but others remain asymptomatic. If untreated, coronary artery disease can lead to MI or a heart attack.
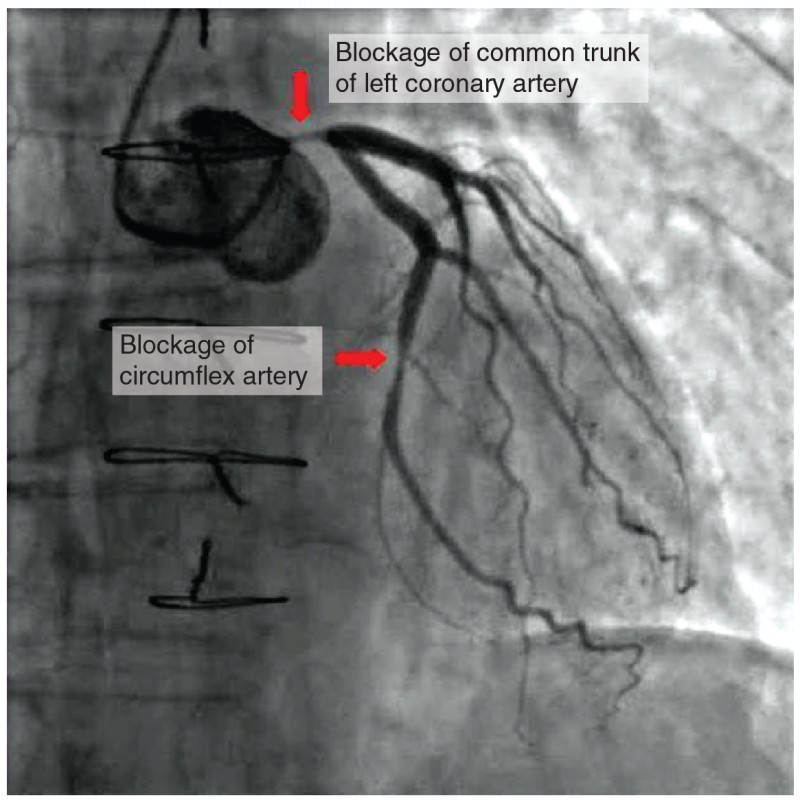
Figure 16. In this coronary angiogram (X-ray), the dye makes visible two occluded coronary arteries. Such blockages can lead to decreased blood flow (ischemia) and insufficient oxygen (hypoxia) delivered to the cardiac tissues. If uncorrected, this can lead to cardiac muscle death (myocardial infarction).
The disease progresses slowly and often begins in children and can be seen as fatty “streaks” in the vessels. It then gradually progresses throughout life. Well-documented risk factors include smoking, family history, hypertension, obesity, diabetes, high alcohol consumption, lack of exercise, stress, and hyperlipidemia or high circulating levels of lipids in the blood. Treatments may include medication, changes to diet and exercise, angioplasty with a balloon catheter, insertion of a stent, or coronary bypass procedure.
Angioplasty is a procedure in which the occlusion is mechanically widened with a balloon. A specialized catheter with an expandable tip is inserted into a superficial vessel, normally in the leg, and then directed to the site of the occlusion. At this point, the balloon is inflated to compress the plaque material and to open the vessel to increase blood flow. Then, the balloon is deflated and retracted. A stent consisting of a specialized mesh is typically inserted at the site of occlusion to reinforce the weakened and damaged walls. Stent insertions have been routine in cardiology for more than 40 years.
Coronary bypass surgery may also be performed. This surgical procedure grafts a replacement vessel obtained from another, less vital portion of the body to bypass the occluded area. This procedure is clearly effective in treating patients experiencing a MI, but overall does not increase longevity. Nor does it seem advisable in patients with stable although diminished cardiac capacity since frequently loss of mental acuity occurs following the procedure. Long-term changes to behavior, emphasizing diet and exercise plus a medicine regime tailored to lower blood pressure, lower cholesterol and lipids, and reduce clotting are equally as effective.