A full-term pregnancy lasts approximately 270 days (approximately 38.5 weeks) from conception to birth. Because it is easier to remember the first day of the last menstrual period (LMP) than to estimate the date of conception, obstetricians set the due date as 284 days (approximately 40.5 weeks) from the LMP. This assumes that conception occurred on day 14 of the woman’s cycle, which is usually a good approximation. The 40 weeks of an average pregnancy are usually discussed in terms of three trimesters, each approximately 13 weeks. During the second and third trimesters, the pre-pregnancy uterus—about the size of a fist—grows dramatically to contain the fetus, causing a number of anatomical changes in the mother.

Figure 1. The uterus grows throughout pregnancy to accommodate the fetus.
Effects of Hormones
Virtually all of the effects of pregnancy can be attributed in some way to the influence of hormones—particularly estrogens, progesterone, and hCG. During weeks 7–12 from the LMP, the pregnancy hormones are primarily generated by the corpus luteum. Progesterone secreted by the corpus luteum stimulates the production of decidual cells of the endometrium that nourish the blastocyst before placentation. As the placenta develops and the corpus luteum degenerates during weeks 12–17, the placenta gradually takes over as the endocrine organ of pregnancy.
The placenta converts weak androgens secreted by the maternal and fetal adrenal glands to estrogens, which are necessary for pregnancy to progress. Estrogen levels climb throughout the pregnancy, increasing 30-fold by childbirth. Estrogens have the following actions:
- They suppress FSH and LH production, effectively preventing ovulation. (This function is the biological basis of hormonal birth control pills.)
- They induce the growth of fetal tissues and are necessary for the maturation of the fetal lungs and liver.
- They promote fetal viability by regulating progesterone production and triggering fetal synthesis of cortisol, which helps with the maturation of the lungs, liver, and endocrine organs such as the thyroid gland and adrenal gland.
- They stimulate maternal tissue growth, leading to uterine enlargement and mammary duct expansion and branching.
Relaxin, another hormone secreted by the corpus luteum and then by the placenta, helps prepare the mother’s body for childbirth. It increases the elasticity of the symphysis pubis joint and pelvic ligaments, making room for the growing fetus and allowing expansion of the pelvic outlet for childbirth. Relaxin also helps dilate the cervix during labor.
The placenta takes over the synthesis and secretion of progesterone throughout pregnancy as the corpus luteum degenerates. Like estrogen, progesterone suppresses FSH and LH. It also inhibits uterine contractions, protecting the fetus from preterm birth. This hormone decreases in late gestation, allowing uterine contractions to intensify and eventually progress to true labor. The placenta also produces hCG. In addition to promoting survival of the corpus luteum, hCG stimulates the male fetal gonads to secrete testosterone, which is essential for the development of the male reproductive system.
The anterior pituitary enlarges and ramps up its hormone production during pregnancy, raising the levels of thyrotropin, prolactin, and adrenocorticotropic hormone (ACTH). Thyrotropin, in conjunction with placental hormones, increases the production of thyroid hormone, which raises the maternal metabolic rate. This can markedly augment a pregnant woman’s appetite and cause hot flashes. Prolactin stimulates enlargement of the mammary glands in preparation for milk production. ACTH stimulates maternal cortisol secretion, which contributes to fetal protein synthesis. In addition to the pituitary hormones, increased parathyroid levels mobilize calcium from maternal bones for fetal use.
Weight Gain
The second and third trimesters of pregnancy are associated with dramatic changes in maternal anatomy and physiology. The most obvious anatomical sign of pregnancy is the dramatic enlargement of the abdominal region, coupled with maternal weight gain. This weight results from the growing fetus as well as the enlarged uterus, amniotic fluid, and placenta. Additional breast tissue and dramatically increased blood volume also contribute to weight gain. Surprisingly, fat storage accounts for only approximately 2.3 kg (5 lbs) in a normal pregnancy and serves as a reserve for the increased metabolic demand of breastfeeding.
During the first trimester, the mother does not need to consume additional calories to maintain a healthy pregnancy. However, a weight gain of approximately 0.45 kg (1 lb) per month is common. During the second and third trimesters, the mother’s appetite increases, but it is only necessary for her to consume an additional 300 calories per day to support the growing fetus. Most women gain approximately 0.45 kg (1 lb) per week.
| Table 1. Contributors to Weight Gain During Pregnancy | ||
|---|---|---|
| Component | Weight (kg) | Weight (lb) |
| Fetus | 3.2–3.6 | 7–8 |
| Placenta and fetal membranes | 0.9–1.8 | 2–4 |
| Amniotic fluid | 0.9–1.4 | 2–3 |
| Breast tissue | 0.9–1.4 | 2–3 |
| Blood | 1.4 | 4 |
| Fat | 0.9–4.1 | 3–9 |
| Uterus | 0.9–2.3 | 2–5 |
| Total | 10–16.3 | 22–36 |
Physiology of Labor
Childbirth, or parturition, typically occurs within a week of a woman’s due date, unless the woman is pregnant with more than one fetus, which usually causes her to go into labor early. As a pregnancy progresses into its final weeks, several physiological changes occur in response to hormones that trigger labor.
First, recall that progesterone inhibits uterine contractions throughout the first several months of pregnancy. As the pregnancy enters its seventh month, progesterone levels plateau and then drop. Estrogen levels, however, continue to rise in the maternal circulation. The increasing ratio of estrogen to progesterone makes the myometrium (the uterine smooth muscle) more sensitive to stimuli that promote contractions (because progesterone no longer inhibits them). Moreover, in the eighth month of pregnancy, fetal cortisol rises, which boosts estrogen secretion by the placenta and further overpowers the uterine-calming effects of progesterone. Some women may feel the result of the decreasing levels of progesterone in late pregnancy as weak and irregular peristaltic Braxton Hicks contractions, also called false labor. These contractions can often be relieved with rest or hydration.
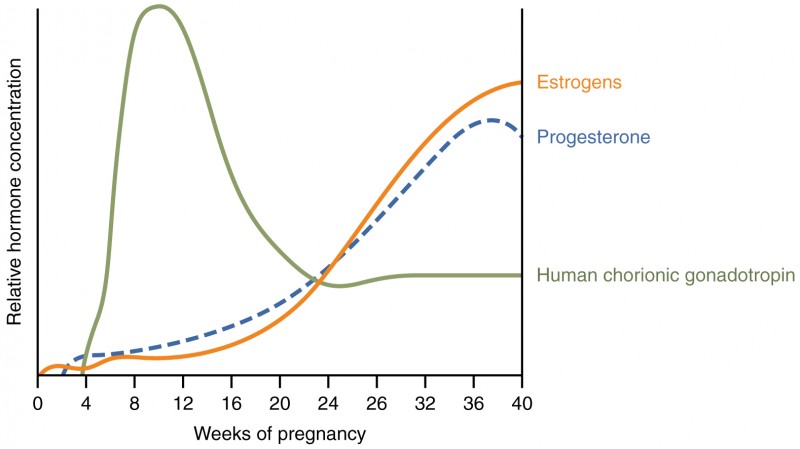
Figure 3. A positive feedback loop of hormones works to initiate labor.
A common sign that labor will be short is the so-called “bloody show.” During pregnancy, a plug of mucus accumulates in the cervical canal, blocking the entrance to the uterus. Approximately 1–2 days prior to the onset of true labor, this plug loosens and is expelled, along with a small amount of blood.
Meanwhile, the posterior pituitary has been boosting its secretion of oxytocin, a hormone that stimulates the contractions of labor. At the same time, the myometrium increases its sensitivity to oxytocin by expressing more receptors for this hormone. As labor nears, oxytocin begins to stimulate stronger, more painful uterine contractions, which—in a positive feedback loop—stimulate the secretion of prostaglandins from fetal membranes. Like oxytocin, prostaglandins also enhance uterine contractile strength. The fetal pituitary also secretes oxytocin, which increases prostaglandins even further. Given the importance of oxytocin and prostaglandins to the initiation and maintenance of labor, it is not surprising that, when a pregnancy is not progressing to labor and needs to be induced, a pharmaceutical version of these compounds (called pitocin) is administered by intravenous drip.
Finally, stretching of the myometrium and cervix by a full-term fetus in the vertex (head-down) position is regarded as a stimulant to uterine contractions. The sum of these changes initiates the regular contractions known as true labor, which become more powerful and more frequent with time. The pain of labor is attributed to myometrial hypoxia during uterine contractions.
Stages of Childbirth
The process of childbirth can be divided into three stages: cervical dilation, expulsion of the newborn, and afterbirth.
Cervical Dilation
For vaginal birth to occur, the cervix must dilate fully to 10 cm in diameter—wide enough to deliver the newborn’s head. The dilation stage is the longest stage of labor and typically takes 6–12 hours. However, it varies widely and may take minutes, hours, or days, depending in part on whether the mother has given birth before; in each subsequent labor, this stage tends to be shorter.
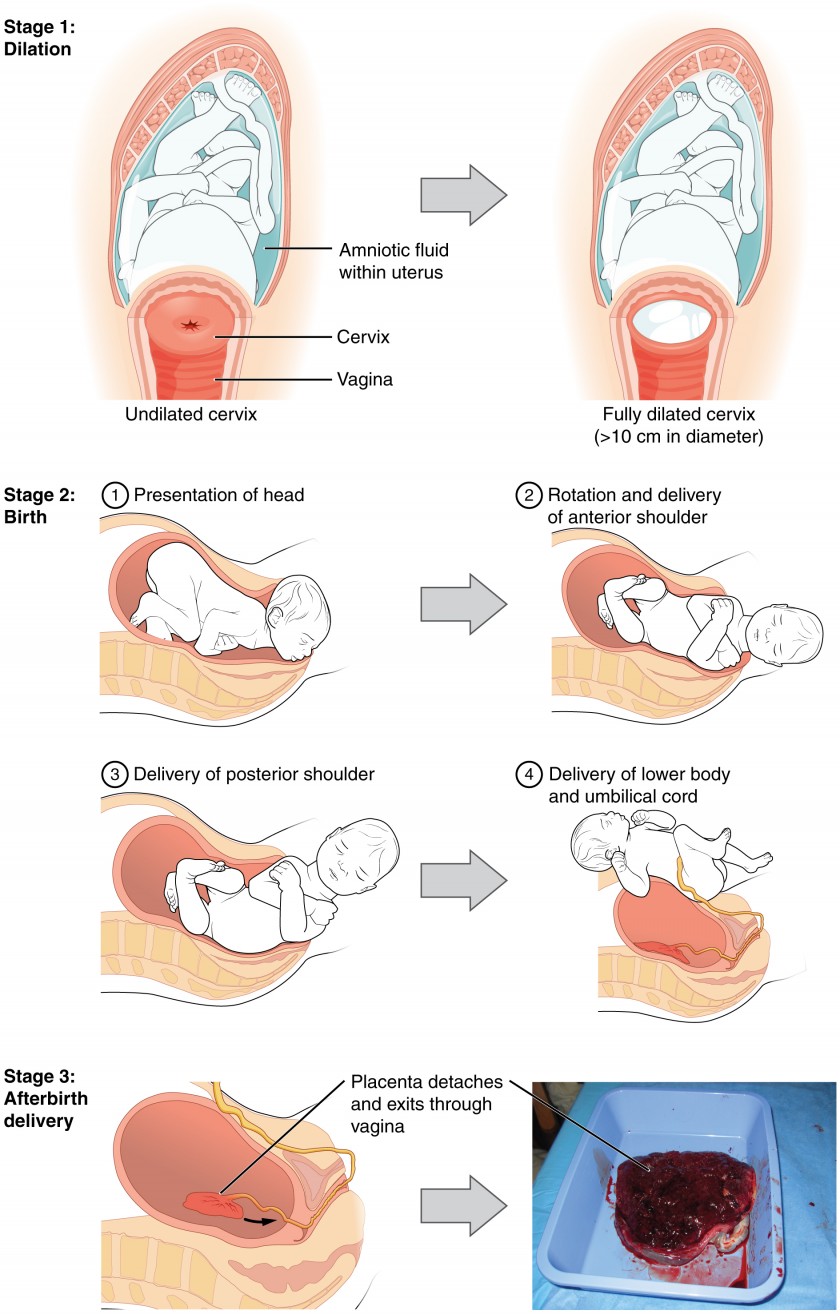
Figure 4. Click for a larger image. The stages of childbirth include Stage 1, early cervical dilation; Stage 2, full dilation and expulsion of the newborn; and Stage 3, delivery of the placenta and associated fetal membranes. (The position of the newborn’s shoulder is described relative to the mother.)
True labor progresses in a positive feedback loop in which uterine contractions stretch the cervix, causing it to dilate and efface, or become thinner. Cervical stretching induces reflexive uterine contractions that dilate and efface the cervix further. In addition, cervical dilation boosts oxytocin secretion from the pituitary, which in turn triggers more powerful uterine contractions. When labor begins, uterine contractions may occur only every 3–30 minutes and last only 20–40 seconds; however, by the end of this stage, contractions may occur as frequently as every 1.5–2 minutes and last for a full minute.
Each contraction sharply reduces oxygenated blood flow to the fetus. For this reason, it is critical that a period of relaxation occur after each contraction. Fetal distress, measured as a sustained decrease or increase in the fetal heart rate, can result from severe contractions that are too powerful or lengthy for oxygenated blood to be restored to the fetus. Such a situation can be cause for an emergency birth with vacuum, forceps, or surgically by Caesarian section.
The amniotic membranes rupture before the onset of labor in about 12 percent of women; they typically rupture at the end of the dilation stage in response to excessive pressure from the fetal head entering the birth canal.
Expulsion Stage
The expulsion stage begins when the fetal head enters the birth canal and ends with birth of the newborn. It typically takes up to 2 hours, but it can last longer or be completed in minutes, depending in part on the orientation of the fetus. The vertex presentation known as the occiput anterior vertex is the most common presentation and is associated with the greatest ease of vaginal birth. The fetus faces the maternal spinal cord and the smallest part of the head (the posterior aspect called the occiput) exits the birth canal first.
In fewer than 5 percent of births, the infant is oriented in the breech presentation, or buttocks down. In a complete breech, both legs are crossed and oriented downward. In a frank breech presentation, the legs are oriented upward. Before the 1960s, it was common for breech presentations to be delivered vaginally. Today, most breech births are accomplished by Caesarian section.
Vaginal birth is associated with significant stretching of the vaginal canal, the cervix, and the perineum. Until recent decades, it was routine procedure for an obstetrician to numb the perineum and perform an episiotomy, an incision in the posterior vaginal wall and perineum. The perineum is now more commonly allowed to tear on its own during birth. Both an episiotomy and a perineal tear need to be sutured shortly after birth to ensure optimal healing. Although suturing the jagged edges of a perineal tear may be more difficult than suturing an episiotomy, tears heal more quickly, are less painful, and are associated with less damage to the muscles around the vagina and rectum.
Upon birth of the newborn’s head, an obstetrician will aspirate mucus from the mouth and nose before the newborn’s first breath. Once the head is birthed, the rest of the body usually follows quickly. The umbilical cord is then double-clamped, and a cut is made between the clamps. This completes the second stage of childbirth.
Afterbirth
The delivery of the placenta and associated membranes, commonly referred to as the afterbirth, marks the final stage of childbirth. After expulsion of the newborn, the myometrium continues to contract. This movement shears the placenta from the back of the uterine wall. It is then easily delivered through the vagina. Continued uterine contractions then reduce blood loss from the site of the placenta. Delivery of the placenta marks the beginning of the postpartum period—the period of approximately 6 weeks immediately following childbirth during which the mother’s body gradually returns to a non-pregnant state. If the placenta does not birth spontaneously within approximately 30 minutes, it is considered retained, and the obstetrician may attempt manual removal. If this is not successful, surgery may be required.
It is important that the obstetrician examines the expelled placenta and fetal membranes to ensure that they are intact. If fragments of the placenta remain in the uterus, they can cause postpartum hemorrhage. Uterine contractions continue for several hours after birth to return the uterus to its pre-pregnancy size in a process called involution, which also allows the mother’s abdominal organs to return to their pre-pregnancy locations. Breastfeeding facilitates this process.
Although postpartum uterine contractions limit blood loss from the detachment of the placenta, the mother does experience a postpartum vaginal discharge called lochia. This is made up of uterine lining cells, erythrocytes, leukocytes, and other debris. Thick, dark, lochia rubra (red lochia) typically continues for 2–3 days, and is replaced by lochia serosa, a thinner, pinkish form that continues until about the tenth postpartum day. After this period, a scant, creamy, or watery discharge called lochia alba (white lochia) may continue for another 1–2 weeks.
Lactation is the process by which milk is synthesized and secreted from the mammary glands of the postpartum female breast in response to an infant sucking at the nipple. Breast milk provides ideal nutrition and passive immunity for the infant, encourages mild uterine contractions to return the uterus to its pre-pregnancy size (i.e., involution), and induces a substantial metabolic increase in the mother, consuming the fat reserves stored during pregnancy.
Structure of the Lactating Breast
Mammary glands are modified sweat glands. The non-pregnant and non-lactating female breast is composed primarily of adipose and collagenous tissue, with mammary glands making up a very minor proportion of breast volume. The mammary gland is composed of milk-transporting lactiferous ducts, which expand and branch extensively during pregnancy in response to estrogen, growth hormone, cortisol, and prolactin. Moreover, in response to progesterone, clusters of breast alveoli bud from the ducts and expand outward toward the chest wall. Breast alveoli are balloon-like structures lined with milk-secreting cuboidal cells, or lactocytes, that are surrounded by a net of contractile myoepithelial cells. Milk is secreted from the lactocytes, fills the alveoli, and is squeezed into the ducts. Clusters of alveoli that drain to a common duct are called lobules; the lactating female has 12–20 lobules organized radially around the nipple. Milk drains from lactiferous ducts into lactiferous sinuses that meet at 4 to 18 perforations in the nipple, called nipple pores. The small bumps of the areola (the darkened skin around the nipple) are called Montgomery glands. They secrete oil to cleanse the nipple opening and prevent chapping and cracking of the nipple during breastfeeding.
The Process of Lactation
The pituitary hormone prolactin is instrumental in the establishment and maintenance of breast milk supply. It also is important for the mobilization of maternal micronutrients for breast milk.
Near the fifth week of pregnancy, the level of circulating prolactin begins to increase, eventually rising to approximately 10–20 times the pre-pregnancy concentration. We noted earlier that, during pregnancy, prolactin and other hormones prepare the breasts anatomically for the secretion of milk. The level of prolactin plateaus in late pregnancy, at a level high enough to initiate milk production. However, estrogen, progesterone, and other placental hormones inhibit prolactin-mediated milk synthesis during pregnancy. It is not until the placenta is expelled that this inhibition is lifted and milk production commences.
After childbirth, the baseline prolactin level drops sharply, but it is restored for a 1-hour spike during each feeding to stimulate the production of milk for the next feeding. With each prolactin spike, estrogen and progesterone also increase slightly.
When the infant suckles, sensory nerve fibers in the areola trigger a neuroendocrine reflex that results in milk secretion from lactocytes into the alveoli. The posterior pituitary releases oxytocin, which stimulates myoepithelial cells to squeeze milk from the alveoli so it can drain into the lactiferous ducts, collect in the lactiferous sinuses, and discharge through the nipple pores. It takes less than 1 minute from the time when an infant begins suckling (the latent period) until milk is secreted (the let-down). The image below summarizes the positive feedback loop of the let-down reflex.

Figure 5. Click for a larger image. A positive feedback loop ensures continued milk production as long as the infant continues to breastfeed.
The prolactin-mediated synthesis of milk changes with time. Frequent milk removal by breastfeeding (or pumping) will maintain high circulating prolactin levels for several months. However, even with continued breastfeeding, baseline prolactin will decrease over time to its pre-pregnancy level. In addition to prolactin and oxytocin, growth hormone, cortisol, parathyroid hormone, and insulin contribute to lactation, in part by facilitating the transport of maternal amino acids, fatty acids, glucose, and calcium to breast milk.
In the final weeks of pregnancy, the alveoli swell with colostrum, a thick, yellowish substance that is high in protein but contains less fat and glucose than mature breast milk. Before childbirth, some women experience leakage of colostrum from the nipples. In contrast, mature breast milk does not leak during pregnancy and is not secreted until several days after childbirth.
| Table 1. Compositions of Human Colostrum, Mature Breast Milk, and Cow’s Milk (g/L) | |||
|---|---|---|---|
| Human colostrum | Human breast milk | Cow’s milk* | |
| Total protein | 23 | 11 | 31 |
| Immunoglobulins | 19 | 0.1 | 1 |
| Fat | 30 | 45 | 38 |
| Lactose | 57 | 71 | 47 |
| Calcium | 0.5 | 0.3 | 1.4 |
| Phosphorus | 0.16 | 0.14 | 0.90 |
| Sodium | 0.50 | 0.15 | 0.41 |
| *Cow’s milk should never be given to an infant. Its composition is not suitable and its proteins are difficult for the infant to digest. | |||
Colostrum is secreted during the first 48–72 hours postpartum. Only a small volume of colostrum is produced—approximately 3 ounces in a 24-hour period—but it is sufficient for the newborn in the first few days of life. Colostrum is rich with immunoglobulins, which confer gastrointestinal, and also likely systemic, immunity as the newborn adjusts to a nonsterile environment.
After about the third postpartum day, the mother secretes transitional milk that represents an intermediate between mature milk and colostrum. This is followed by mature milk from approximately postpartum day 10. As you can see in the accompanying table, cow’s milk is not a substitute for breast milk. It contains less lactose, less fat, and more protein and minerals. Moreover, the proteins in cow’s milk are difficult for an infant’s immature digestive system to metabolize and absorb.
The first few weeks of breastfeeding may involve leakage, soreness, and periods of milk engorgement as the relationship between milk supply and infant demand becomes established. Once this period is complete, the mother will produce approximately 1.5 liters of milk per day for a single infant, and more if she has twins or triplets. As the infant goes through growth spurts, the milk supply constantly adjusts to accommodate changes in demand. A woman can continue to lactate for years, but once breastfeeding is stopped for approximately 1 week, any remaining milk will be reabsorbed; in most cases, no more will be produced, even if suckling or pumping is resumed.
Mature milk changes from the beginning to the end of a feeding. The early milk, called foremilk, is watery, translucent, and rich in lactose and protein. Its purpose is to quench the infant’s thirst. Hindmilk is delivered toward the end of a feeding. It is opaque, creamy, and rich in fat, and serves to satisfy the infant’s appetite.
During the first days of a newborn’s life, it is important for meconium to be cleared from the intestines and for bilirubin to be kept low in the circulation. Recall that bilirubin, a product of erythrocyte breakdown, is processed by the liver and secreted in bile. It enters the gastrointestinal tract and exits the body in the stool. Breast milk has laxative properties that help expel meconium from the intestines and clear bilirubin through the excretion of bile. A high concentration of bilirubin in the blood causes jaundice. Some degree of jaundice is normal in newborns, but a high level of bilirubin—which is neurotoxic—can cause brain damage. Newborns, who do not yet have a fully functional blood–brain barrier, are highly vulnerable to the bilirubin circulating in the blood. Indeed, hyperbilirubinemia, a high level of circulating bilirubin, is the most common condition requiring medical attention in newborns. Newborns with hyperbilirubinemia are treated with phototherapy because UV light helps to break down the bilirubin quickly.
Candela Citations
- Anatomy & Physiology. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25