Learning Objectives
By the end of this section, you will be able to:
- Use the ideal gas law to compute gas molar masses
- Perform stoichiometric calculations involving gaseous substances
The study of the chemical behavior of gases was part of the basis of perhaps the most fundamental chemical revolution in history. French nobleman Antoine Lavoisier, widely regarded as the “father of modern chemistry,” changed chemistry from a qualitative to a quantitative science through his work with gases. He discovered the law of conservation of matter, discovered the role of oxygen in combustion reactions, determined the composition of air, explained respiration in terms of chemical reactions, and more. He was a casualty of the French Revolution, guillotined in 1794. Of his death, mathematician and astronomer Joseph-Louis Lagrange said, “It took the mob only a moment to remove his head; a century will not suffice to reproduce it.”
As described in an earlier chapter of this text, we can turn to chemical stoichiometry for answers to many of the questions that ask “How much?” We can answer the question with masses of substances or volumes of solutions. However, we can also answer this question another way: with volumes of gases. We can use the ideal gas equation to relate the pressure, volume, temperature, and number of moles of a gas. Here we will combine the ideal gas equation with other equations to find gas density and molar mass. We will deal with mixtures of different gases, and calculate amounts of substances in reactions involving gases. This section will not introduce any new material or ideas, but will provide examples of applications and ways to integrate concepts we have already discussed.
Molar Mass of a Gas
Another useful application of the ideal gas law involves the determination of molar mass. By definition, the molar mass of a substance is the ratio of its mass in grams, m, to its amount in moles, n
[latex]\large\mathcal{M}=\frac{\text{grams of substance}}{\text{moles of substance}}=\frac{m}{n}[/latex]
The ideal gas equation can be rearranged to isolate n:
[latex]\large n=\frac{PV}{RT}[/latex]
and then combined with the molar mass equation to yield:
[latex]\large\mathcal{M}=\frac{mRT}{PV}[/latex]
This equation can be used to derive the molar mass of a gas from measurements of its pressure, volume, temperature, and mass.
Example 1: Determining the Molar Mass of a Volatile Liquid
The approximate molar mass of a volatile liquid can be determined by:
- Heating a sample of the liquid in a flask with a tiny hole at the top, which converts the liquid into gas that may escape through the hole
- Removing the flask from heat at the instant when the last bit of liquid becomes gas, at which time the flask will be filled with only gaseous sample at ambient pressure
- Sealing the flask and permitting the gaseous sample to condense to liquid, and then weighing the flask to determine the sample’s mass (see Figure 1)
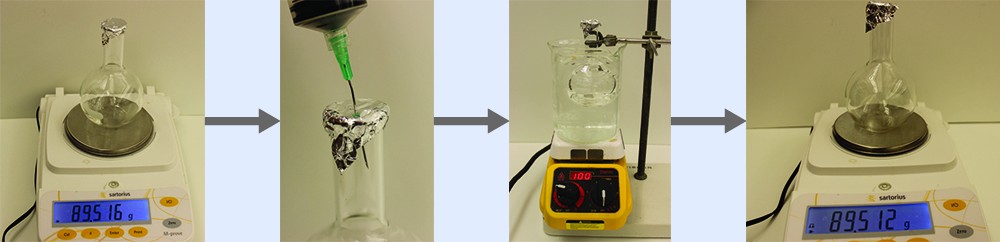
Using this procedure, a sample of chloroform gas weighing 0.494 g is collected in a flask with a volume of 129 cm3 at 99.6 °C when the atmospheric pressure is 742.1 mm Hg. What is the approximate molar mass of chloroform?
Check Your Learning
A sample of phosphorus that weighs 3.243 × 10-2 g exerts a pressure of 31.89 kPa in a 56.0 mL bulb at 550 °C. What is the molar mass of phosphorus vapor?
Chemical Stoichiometry and Gases
Chemical stoichiometry describes the quantitative relationships between reactants and products in chemical reactions.
We have previously measured quantities of reactants and products using masses for solids; now we can also use gas volumes to indicate quantities. If we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate how many moles of the gas are present. If we know how many moles of a gas are involved, we can calculate the volume of a gas at any temperature and pressure.
Example 2: Gas Stoichiometry
What volume of hydrogen at 27 °C and 723 torr may be prepared by the reaction of 8.88 g of gallium with an excess of hydrochloric acid?
[latex]\large2\text{ Ga}\left(s\right)+6\text{ HCl}\left(aq\right)\rightarrow 2{\text{ GaCl}}_{3}\left(aq\right)+3{\text{ H}}_{2}\left(g\right)[/latex]
Check Your Learning
Sulfur dioxide is an intermediate in the preparation of sulfuric acid. What volume of SO2 at 343 °C and 1.21 atm is produced by burning 1.00 kg of sulfur in oxygen?
[latex]\large\text{S}_{8}\left(s\right)+\text{8 O}_{2}\left(g\right)\rightarrow {\text{8 SO}}_{2}\left(g\right)[/latex]
Example 3: Gas Stoichiometry
How many grams of Zn is required in the following reaction to produce 19.6 L of hydrogen gas at 299 K and 1.07 atm?
[latex]\large \text{Zn}\left(s\right)+2\text{ HCl}\left(aq\right)\rightarrow 2{\text{ ZnCl}}_{2}\left(aq\right)+{\text{H}}_{2}\left(g\right)[/latex]
Check Your Learning
What pressure of HCl is generated if 3.44 g of Cl2 are reacted in 4.55 L at 455 K?
[latex]\large {\text{H}}_{2}\left(g\right)+{\text{Cl}}_{2}\left(g\right)\rightarrow 2{\text{ HCl}}\left(g\right)[/latex]
Key Concepts and Summary
The ideal gas law can be used to derive a number of convenient equations relating directly measured quantities to properties of interest for gaseous substances and mixtures. Appropriate rearrangement of the ideal gas equation may be made to permit the calculation of gas molar masses. The ideal gas law can also be used in relation with stoichiometry.
Exercises
- What is the molar mass of a gas if 0.0494 g of the gas occupies a volume of 0.100 L at a temperature 26 °C and a pressure of 307 torr?
- What is the molar mass of a gas if 0.281 g of the gas occupies a volume of 125 mL at a temperature 126 °C and a pressure of 777 torr?
- Joseph Priestley first prepared pure oxygen by heating mercuric oxide, HgO: [latex]2\text{ HgO}\left(s\right)\rightarrow 2\text{ Hg}\left(l\right)+{\text{ O}}_{2}\left(g\right)[/latex]
- Outline the steps necessary to answer the following question: What volume of O2 at 23° C and 0.975 atm is produced by the decomposition of 5.36 g of HgO?
- Answer the question.
- Cavendish prepared hydrogen in 1766 by the novel method of passing steam through a red-hot gun barrel: [latex]4{\text{ H}}_{2}\text{O}\left(g\right)+3\text{ Fe}\left(s\right)\rightarrow{\text{ Fe}}_{3}{\text{O}}_{4}\left(s\right)+4{\text{ H}}_{2}\left(g\right)[/latex]
- Outline the steps necessary to answer the following question: What volume of H2 at a pressure of 745 torr and a temperature of 20 °C can be prepared from the reaction of 15.O g of H2O?
- Answer the question.
- The chlorofluorocarbon CCl2F2 can be recycled into a different compound by reaction with hydrogen to produce CH2F2(g), a compound useful in chemical manufacturing: [latex]{\text{CCl}}_{2}{\text{F}}_{2}\left(g\right)+4{\text{ H}}_{2}\left(g\right)\rightarrow{\text{CH}}_{2}{\text{F}}_{2}\left(g\right)+2\text{ HCl}\left(g\right).[/latex]
- Outline the steps necessary to answer the following question: What volume of hydrogen at 225 atm and 35.5 °C would be required to react with 1 ton (1.000 × 103 kg) of CCl2F2?
- Answer the question.
- Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (NaN3). The other product is sodium metal. Calculate the volume of nitrogen gas at 27 °C and 756 torr formed by the decomposition of 125 g of sodium azide.
- Lime, CaO, is produced by heating calcium carbonate, CaCO3; carbon dioxide is the other product.
- Outline the steps necessary to answer the following question: What volume of carbon dioxide at 875 °C and 0.966 atm is produced by the decomposition of 1 ton (1.000 × 103 kg) of calcium carbonate?
- Answer the question.
- Before small batteries were available, carbide lamps were used for bicycle lights. Acetylene gas, C2H2, and solid calcium hydroxide were formed by the reaction of calcium carbide, CaC2, with water. The ignition of the acetylene gas provided the light. Currently, the same lamps are used by some cavers, and calcium carbide is used to produce acetylene for carbide cannons.
- Outline the steps necessary to answer the following question: What volume of C2H2 at 1.005 atm and 12.2 °C is formed by the reaction of 15.48 g of CaC2 with water?
- Answer the question.
- Calculate the volume of oxygen required to burn 12.00 L of ethane gas, C2H6, to produce carbon dioxide and water, if the volumes of C2H6 and O2 are measured under the same conditions of temperature and pressure.
- What volume of O2 at STP is required to oxidize 8.0 L of NO at STP to NO2? What volume of NO2 is produced at STP?
Candela Citations
- Introductory Chemistry- 1st Canadian Edition . Authored by: Jessie A. Key and David W. Ball. Provided by: BCCampus. Located at: https://opentextbc.ca/introductorychemistry/. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike. License Terms: Download this book for free at http://open.bccampus.ca
- Chemistry. Provided by: OpenStaxCollege. Located at: http://openstaxcollege.org. License: CC BY: Attribution. License Terms: Download for free at https://openstaxcollege.org/textbooks/chemistry/get
