Objectives
After completing this section, you should be able to
- recognize that atoms other than carbon can be chiral centres.
- explain why enantiomers of compounds such as ethylmethylamine cannot normally be isolated.
Study Notes
The first example of a resolvable compound containing a chiral nitrogen atom was resolved by William Pope and Stanley Peachey in 1899. It had the structure shown below.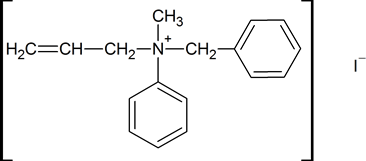
Chiral sulfoxides find application in certain drugs such as esomeprazole and armodafinil and are a good example of a stereogenic sulfur center.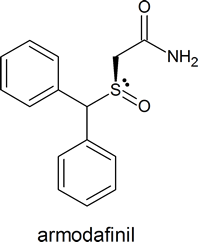
Stereogenic Nitrogen
Single-bonded nitrogen is pyramidal in shape, with the non-bonding electron pair pointing to the unoccupied corner of a tetrahedral region. Since the nitrogen in these compounds is bonded to three different groups, its configuration is chiral. The non-identical mirror-image configurations are illustrated in the following diagram (the remainder of the molecule is represented by R, and the electron pair is colored yellow). If these configurations were stable, there would be four additional stereoisomers of ephedrine and pseudoephedrine. However, pyramidal nitrogen is normally not configurationally stable. It rapidly inverts its configuration (equilibrium arrows) by passing through a planar, sp2-hybridized transition state, leading to a mixture of interconverting R and S configurations. If the nitrogen atom were the only chiral center in the molecule, a 50:50 (racemic) mixture of R and S configurations would exist at equilibrium. If other chiral centers are present, as in the ephedrin isomers, a mixture of diastereomers will result. The take-home message is that nitrogen does not contribute to isolable stereoisomers.

Asymmetric quaternary ammonium groups are also chiral. Amines, however, are not chiral, because they rapidly invert, or turn ‘inside out’, at room temperature.

The phosphorus center of phosphate ion and organic phosphate esters, for example, is tetrahedral, and thus is potentially a stereocenter.

We will see in chapter 10 how researchers, in order to investigate the stereochemistry of reactions at the phosphate center, incorporated sulfur and/or 17O and 18O isotopes of oxygen (the ‘normal’ isotope is 16O) to create chiral phosphate groups. Phosphate triesters are chiral if the three substituent groups are different.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Prof. Steven Farmer (Sonoma State University)
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)