Hybrid Orbitals and the Structure of Ethane
Objective
After completing this section, you should be able to describe the structure of ethane in terms of the sp3 hybridization of the two carbon atoms present in the molecule.
Bonding in Ethane
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp3 orbital from each of the carbons, while the six carbon-hydrogen bonds are formed from overlaps between the remaining sp3 orbitals on the two carbons and the 1s orbitals of hydrogen atoms. All of these are sigma bonds.

Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. This means, in the case of ethane molecule, that the two methyl (CH3) groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other.
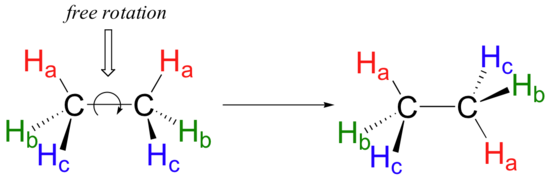
In chapter 3 we will learn more about the implications of rotational freedom in sigma bonds, when we discuss the ‘conformation’ of organic molecules.
The sp3 bonding picture is also used to described the bonding in amines, including ammonia, the simplest amine. Just like the carbon atom in methane, the central nitrogen in ammonia is sp3-hybridized. With nitrogen, however, there are five rather than four valence electrons to account for, meaning that three of the four hybrid orbitals are half-filled and available for bonding, while the fourth is fully occupied by a (non-bonding) pair of electrons.
C2H4, also known as ethylene or ethene, is a gaseous material created synthetically through steam cracking. In nature, it is released in trace amounts by plants to signal their fruits to ripen. Ethene consists of two sp2-hybridized carbon atoms, which are sigma bonded to each other and to two hydrogen atoms each. The remaining unhybridized p orbitals on the carbon form a pi bond, which gives ethene its reactivity.
Example
Draw pentane, CH3CH2CH2CH2CH3, predict the bond angles within this molecule.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
