Objectives
After completing this section, you should be able to account for the stereochemistry of the product of the addition of water to an alkene in terms of the formation of a planar carbocation.
Study Notes
Organic reactions in the laboratory or in living systems can produce chiral centres. Consider reaction of 1-butene with water (acid catalyzed). Markovnikov regiochemistry occurs and the OH adds to the second carbon. However, both R and S products occur giving a racemic (50/50) mixture of 2‑butanol. How does this occur? The proton addition to 1‑butene results in a planar carbocation intermediate. A molecule of water is then equally likely to attack from the top or the bottom of this cation to produce either (S)‑2‑butanol or (R)‑2‑butanol, respectively.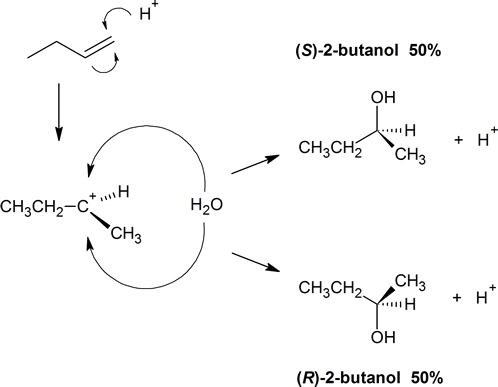
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)