Learning Objectives
After completing this section, you should be able to
- provide the correct IUPAC name for any given alkane structure (Kekulé, condensed or shorthand).
- draw the Kekulé, condensed or shorthand structure of an alkane, given its IUPAC name.
Key Terms
Make certain that you can define, and use in context, the key term below.
- IUPAC system
Study Notes
The IUPAC system of nomenclature aims to ensure
- that every organic compound has a unique, unambiguous name.
- that the IUPAC name of any compound conveys the structure of that compound to a person familiar with the system.
One way of checking whether the name you have given to an alkane is reasonable is to count the number of carbon atoms implied by the chosen name. For example, if you named a compound 3‑ethyl-4‑methylheptane, you have indicated that the compound contains a total of 10 carbon atoms—seven carbon atoms in the main chain, two carbon atoms in an ethyl group, and one carbon atom in a methyl group. If you were to check the given structure and find 11 carbon atoms, you would know that you had made a mistake. Perhaps the name you should have written was 3‑ethyl-4,4‑dimethylheptane!
When naming alkanes, a common error of beginning students is a failure to pick out the longest carbon chain. For example, the correct name for the compound shown below is 3‑methylheptane, not 2‑ethylhexane.
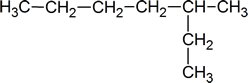
Remember that every substituent must have a number, and do not forget the prefixes: di, tri, tetra, etc.
You must use commas to separate numbers, and hyphens to separate numbers and substituents. Notice that 3‑methylhexane is one word.
Hydrocarbons
The simplest class of organic compounds is the hydrocarbons, which consist entirely of carbon and hydrogen. Petroleum and natural gas are complex, naturally occurring mixtures of many different hydrocarbons that furnish raw materials for the chemical industry. The four major classes of hydrocarbons are the following: the alkanes, which contain only carbon–hydrogen and carbon–carbon single bonds; the alkenes, which contain at least one carbon–carbon double bond; the alkynes, which contain at least one carbon–carbon triple bond; and the aromatic hydrocarbons, which usually contain rings of six carbon atoms that can be drawn with alternating single and double bonds. Alkanes are also called saturated hydrocarbons, whereas hydrocarbons that contain multiple bonds (alkenes, alkynes, and aromatics) are unsaturated.
Alkanes

Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.
The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10. A common “ane” suffix identifies these compounds as alkanes. Longer chain alkanes are well known, and their names may be found in many reference and text books. The names methane through decane should be memorized, since they constitute the root of many IUPAC names. Fortunately, common numerical prefixes are used in naming chains of five or more carbon atoms.
| Name | Molecular Formula |
Structural Formula |
Isomers | Name | Molecular Formula |
Structural Formula |
Isomers | |
|---|---|---|---|---|---|---|---|---|
| methane | CH4 | CH4 | 1 | hexane | C6H14 | CH3(CH2)4CH3 | 5 | |
| ethane | C2H6 | CH3CH3 | 1 | heptane | C7H16 | CH3(CH2)5CH3 | 9 | |
| propane | C3H8 | CH3CH2CH3 | 1 | octane | C8H18 | CH3(CH2)6CH3 | 18 | |
| butane | C4H10 | CH3CH2CH2CH3 | 2 | nonane | C9H20 | CH3(CH2)7CH3 | 35 | |
| pentane | C5H12 | CH3(CH2)3CH3 | 3 | decane | C10H22 | CH3(CH2)8CH3 | 75 |
Some important behavior trends and terminologies
- The formulas and structures of these alkanes increase uniformly by a CH2 increment.
- A uniform variation of this kind in a series of compounds is called homologous.
- These formulas all fit the CnH2n+2 rule (for acyclic aka non-cyclic) alkanes. This is also the highest possible H/C ratio for a stable hydrocarbon.
- Since the H/C ratio in these compounds is at a maximum, we call them saturated (with hydrogen).
Cyclic hydrocarbons
In a cyclic hydrocarbon, the ends of a hydrocarbon chain are connected to form a ring of covalently bonded carbon atoms. Cyclic hydrocarbons are named by attaching the prefix cyclo– to the name of the alkane, the alkene, or the alkyne. The simplest cyclic alkanes are cyclopropane (C3H6) a flammable gas that is also a powerful anesthetic, and cyclobutane (C4H8) (part (c) in Figure 3.7.2). The most common way to draw the structures of cyclic alkanes is to sketch a polygon with the same number of vertices as there are carbon atoms in the ring; each vertex represents a CH2 unit. The structures of the cycloalkanes that contain three to six carbon atoms are shown schematically in the figure below:

The simple cycloalkanes
Alkyl groups
Acyclic alkanes can be described by the general formula CnH2n+2. An alkyl group is formed by removing one hydrogen from the alkane chain and is described by the formula CnH2n+1. The removal of this hydrogen results in a stem change from -ane to -yl. Take a look at the following examples.
The same concept can be applied to any of the straight chain alkane names provided in the table above. Examples of some common alkyl groups are given in the following table. Note that the “ane” suffix is replaced by “yl” in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
| Group | CH3– | C2H5– | CH3CH2CH2– | (CH3)2CH– | CH3CH2CH2CH2– | (CH3)2CHCH2– | CH3CH2CH(CH3)– | (CH3)3C– | R– |
|---|---|---|---|---|---|---|---|---|---|
| Name | Methyl | Ethyl | Propyl | Isopropyl | Butyl | Isobutyl | sec-Butyl | tert-Butyl | Alkyl |
Similarly, groups of atoms derived from aromatic hydrocarbons are aryl groups, which sometimes have unexpected names. For example, the –C6H5 fragment is derived from benzene, but it is called a phenyl group. In general formulas and structures, alkyl and aryl groups are often abbreviated as R.
Naming complex alkanes

Beginning with butane (C4H10), and becoming more numerous with larger alkanes, we note the existence of alkane isomers. For example, there are five C6H14 isomers, shown below as abbreviated line formulas (A through E):

Although these distinct compounds all have the same molecular formula, only one (A) can be called hexane. How then are we to name the others? (Answers below.)
The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. Examples of some common alkyl groups are given in the following table. Note that the “ane” suffix is replaced by “yl” in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
IUPAC Rules for Alkane Nomenclature
- Find and name the longest continuous carbon chain.
- Identify and name groups attached to this chain.
- Number the chain consecutively, starting at the end nearest a substituent group.
- Designate the location of each substituent group by an appropriate number and name.
- Assemble the name, listing groups in alphabetical order.
- The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
For the above isomers of hexane the IUPAC names are: B 2-methylpentane C 3-methylpentane D 2,2-dimethylbutane E 2,3-dimethylbutane.
Naming alkyl halides
Halogen substituents are easily accommodated, using the names: fluoro (F-), chloro (Cl-), bromo (Br-) and iodo (I-). These are inserted alphabetically into the name in the same way as alkyl groups are included when naming complex alkanes.
Example: Halogen Substitution
For example, (CH3)2CHCH2CH2Br would be named 1-bromo-3-methylbutane. If the halogen is bonded to a simple alkyl group an alternative “alkyl halide” name may be used. Thus, C2H5Cl may be named chloroethane (no locator number is needed for a two carbon chain) or ethyl chloride.
Example
What is the name of the following molecule?

Example
What is the name of the following molecule?

Example
What is the name of the following molecule?

Exercises
Question
Give the name of the following molecules:

Naming ethers
| Alkyl Group | Name | Alkoxy Group | Name | |
|---|---|---|---|---|
| CH3– | Methyl | CH3O– | Methoxy | |
| CH3CH2– | Ethyl | CH3CH2O– | Ethoxy | |
| (CH3)2CH– | Isopropyl | (CH3)2CHO– | Isopropoxy | |
| (CH3)3C– | tert-Butyl | (CH3)3CO– | tert-Butoxy | |
| C6H5– | Phenyl | C6H5O– | Phenoxy |
Ethers are traditionally named by naming each of the two carbon groups as a separate word followed by a space and the word ether. The IUPAC system names the smaller -OR group as a substituent using the group name, alkoxy.
Example 1
CH3-CH2-O-CH3 is called ethyl methyl ether or methoxyethane.
The smaller, shorter alkyl group becomes the alkoxy substituent. The larger, longer alkyl group side becomes the alkane base name. The alkyl group on each side of the oxygen is numbered separately. The numbering priority is given to the carbon closest to the oxygen. For example, CH3CH2CH2CH2CH2-O-CH2CH2CH3 is 1-propoxypentane. If there is cis or trans stereochemistry, the same rule still applies.
Example
- $$CH_3CH_2OCH_2CH_3$$, diethyl ether (sometimes referred to as just ether)
- $$CH_3OCH_2CH_2OCH_3$$, ethylene glycol dimethyl ether (glyme).

Common names
Simple ethers are given common names in which the alkyl groups bonded to the oxygen are named in alphabetical order followed by the word “ether”. Many simple ethers are symmetrical, in that the two alkyl substituents are the same. These are named as “dialkyl ethers”.
Sulfides
Sulfur analogs of ethers (R–S–R’) are called sulfides (or thioethers), e.g., (CH3)3C–S–CH3 is tert-butyl methyl sulfide. Sulfides are chemically more reactive than ethers.
References
- Schore, Neil E. and Vollhardt, K. Peter C. Organic Chemistry: Structure and Function. New York: Bleyer, Brennan, 2007.
- Winter, Arthur. Organic Chemistry for Dummies. Hoboken, New Jersey: Wiley, 2005.
- Pellegrini, Frank. Cliffs QuickReview Organic Chemistry II. Foster City, CA: Wiley, 2000
Candela Citations
- 3.4 Naming Alkanes. Authored by: Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), Tim Soderbergu00a0(University of Minnesota, Morris). Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_03%3A_Organic_Compounds%3A_Alkanes_and_Their_Stereochemistry/3.4_Naming_Alkanes. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Nomenclature of Ethers. Authored by: William Reusch, Richard Banks. Located at: https://chem.libretexts.org/Core/Organic_Chemistry/Ethers/Nomenclature_of_Ethers. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 3.7: Names of Formulas of Organic Compounds. Located at: https://chem.libretexts.org/Textbook_Maps/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/03%3A_Chemical_Compounds/3.7%3A__Names_of_Formulas_of_Organic_Compounds. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike









