One of the largest and most diverse classes of reactions is composed of nucleophilic additions to a carbonyl group. Conjugation of a double bond to a carbonyl group transmits the electrophilic character of the carbonyl carbon to the beta-carbon of the double bond. These conjugated carbonyl are called enones or α, β unsaturated carbonyls. A resonance description of this transmission is shown below.

From this formula it should be clear that nucleophiles may attack either at the carbonyl carbon, as for any aldehyde, ketone or carboxylic acid derivative, or at the beta-carbon. These two modes of reaction are referred to as 1,2-addition and 1,4-addition respectively. A 1,4-addition is also called a conjugate addition.
Basic reaction of 1,2 addition
Here the nucleophile adds to the carbon which is in the one position. The hydrogen adds to the oxygen which is in the two position.

Basic reaction of 1,4 addition
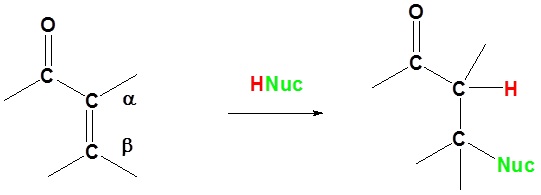
In 1,4 addition the nucleophile is added to the carbon β to the carbonyl while the hydrogen is added to the carbon α to the carbonyl.
Mechanism for 1,4 addition
1) Nucleophilic attack on the carbon β to the carbonyl

2) Proton Transfer

Here we can see why this addition is called 1,4. The nucleophile bonds to the carbon in the one position and the hydrogen adds to the oxygen in the four position.
3) Tautomerization

Going from reactant to products simplified

1,2 vs. 1,4 addition
Whether 1,2 or 1,4-addition occurs depends on multiple variables but mostly it is determined by the nature of the nucleophile. During the addition of a nucleophile there is a competition between 1,2 and 1,4 addition products. If the nucleophile is a strong nucleophile, such as a Grignard reagent, both the 1,2 and 1,4 reactions are irreversible and therefore are under kinetic control. Since 1,2-additions to the carbonyl group are fast, we would expect to find a predominance of 1,2-products from these reactions.
If the nucleophile is a weak base, such as alcohols or amines, then the 1,2 addition is usually reversible. This means the competition between 1,2 and 1,4 addition is under thermodynamic control. In this case 1,4-addition dominates because the stable carbonyl group is retained.
Nucleophiles which add 1,4 to α,β unsaturated carbonyls
Water

Alcohols

Thiols

1o Amines

2o Amines

HBr

Cyanides

Gilman Reagents: These act as a source of R:–

Example

Nucleophiles which add 1,2 to α, β unsaturated carbonyls
Metal Hydrides


Grignard Reagents

Organolithium Reagents

Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Candela Citations
- Conjugate Addition Reactions. Authored by: Prof. Steven Farmer and William Reusch. Located at: https://chem.libretexts.org/?title=Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Aldehydes_and_Ketones/Reactivity_of_Aldehydes_%26_Ketones/Conjugate_Addition_Reactions. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- CO19. Conjugate Addition. Authored by: Chris P Schaller, Ph.D. . Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Addition_Reactions/Addition_to_Carbonyls/CO19._Conjugate_Addition. Project: Chemistry LibreTexts. License: Public Domain: No Known Copyright

