In this reaction three reactions are required.
- A nitration
- A conversion from the nitro group to an amine
- A bromination
Because the end product is meta a meta directing group must be utilized. Of the nitro, bromine, and amine group, only the nitro group is meta direction. This means that the first step need to be the nitration and not the bromination. Also, the conversion of the nitro group to an amine must occur last because the amine group is ortho/para direction.

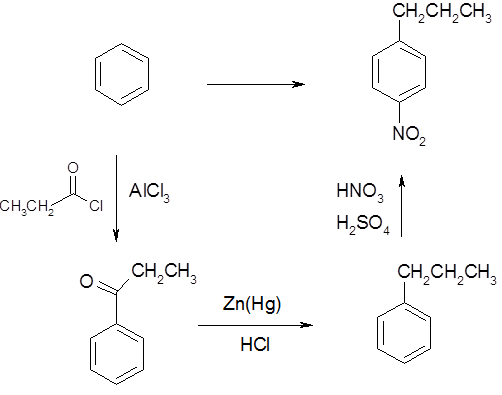
From benzene make p-nitropropylbenzene :
In this reaction three reactions are required.
- A Friedel Crafts acylation
- A conversion from the acyl group to an alkane
- A nitration
Because the propyl group has more than two carbons, it must be added in two steps. A Friedel Crafts acylation followed by a Clemmensen Reduction. Remeber that Friedel Crafts reactions are hindered if the benzene ring is strongly deactivated. This means that the acyl group must go on first. Because the end product is para a para directing group must be utilized. Of the nitro, acyl, and alkane group, only the alkane group is meta direction. This means that the acyl group must be converted to an alkane prior to the nitration step.
Candela Citations
- 18.15 Examples of Multistep Synthesis. Authored by: Prof. Steven Farmer . Provided by: Sonoma State University. Located at: https://chem.libretexts.org/LibreTexts/University_of_Illinois%2C_Springfield/UIS%3A_CHE_269_-_Organic_Chemistry_II_(Morsch)/Chapters/Chapter_18%3A_Electrophilic_Aromatic_Substitution/18.15_Examples_of_Multistep_Synthesis. Project: Chemistry LibreTexts . License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike