key terms
Make certain that you can define, and use in context, the key terms below.
- benzylic oxidation
- benzylic position
- side-chain oxidation
Study Notes
As you can see from the examples, no matter what the length of the alkyl group in the arene substrate, the product is always a one-carbon carboxyl group. Thus, the benzylic carbon atom has been oxidized and the term benzylic oxidation is appropriate. The term side-chain oxidation is also commonly used.
In alkylbenzenes, the carbon atom which is attached to the aromatic ring is particularly reactive. Reactions taking place at this carbon atom are said to occur at the benzylic position.
Benzylic halides undergo the typical reactions of alkyl halides; thus, you can expect to see such compounds used frequently in multistep syntheses.
Note that we have adopted the terminology given below.
Any compound of the type

(where X = halogen) will be referred to as a “benzylic halide.”
Compounds of the type
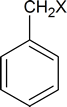
are actually called benzyl chloride, benzyl bromide, etc.
The compound

is called benzyl alcohol.
Oxidation of Alkyl Side-Chains
As noted earlier in section 16.1, alkyl side chains are activated towards oxidation at the benzylic position, because free radicals are stabilized at that position through resonance (see section 16.4.). When heated with aqueous potassium permanganate (KMnO4) under acidic conditions, alkylbenzenes are oxidized to benzoic acids, as long as the alkyl group contains at least one hydrogen at the benzylic position. This means that tert-butylbenzene (shown below) does not react. Aryl methyl ketones (acetophenones), made by the Friedel-Crafts reaction, can also be oxidized to benzoic acids using hot aqueous permanganate.

Bromination of the benzylic Carbon
The benzylic C-H bonds weaker than most sp3 hybridized C-H. This is because the radical formed from homolysis is resonance stabilized, as discussed in section 16.4. We will study radicals in more detail in chapter 18.

Because of the weak C-H bonds, benzylic hydrogens can form benzylic halides under radical conditions.

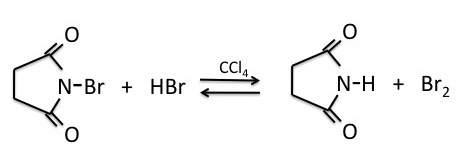
NBS as a Bromine Source
NBS (N-bromosuccinimide) is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts with trace amounts of HBr to produce a low enough concentration of bromine to facilitate the benzylic bromination reaction via a radical process.
Exercises
Questions
Q16.9.1
Predict the products.

Q16.9.2
Consider a benzyl radical. Would it be more stable than an alkyl radical? Explain.
Solutions
S16.9.1
The second one leads to no reaction because it requires a hydrogen on the carbon next to the phenyl ring.

S16.9.2
Yes it would be more stable than an alkyl radical, consider the pi system able to stabilize through resonance.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
Candela Citations
- 16.9: Oxidation of Aromatic Compounds. Authored by: Dr. Dietmar Kennepohl FCIC; Prof. Steven Farmer; William Reusch, professor Emeritus. Provided by: Athabasca University; Sonoma State University; Michigan State. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_16%3A_Chemistry_of_Benzene_-_Electrophilic_Aromatic_Substitution/16.09_Oxidation_of_Aromatic_Compounds. Project: Chemistry LibreTexts . License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 16.9: Oxidation of Aromatic Compounds. Authored by: Dr. Dietmar Kennepohl FCIC; Prof. Steven Farmer; William Reusch, Professor Emeritus. Provided by: Athabasca University; Sonoma State University; Michigan State U. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_16%3A_Chemistry_of_Benzene_-_Electrophilic_Aromatic_Substitution/16.09_Oxidation_of_Aromatic_Compounds. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
