Discuss different components and types of epigenetic gene regulation
Eukaryotic gene expression is more complex than prokaryotic gene expression because the processes of transcription and translation are physically separated. Unlike prokaryotic cells, eukaryotic cells can regulate gene expression at many different levels. Eukaryotic gene expression begins with control of access to the DNA. This form of regulation, called epigenetic regulation, occurs even before transcription is initiated.
Learning Objectives
- Explain the process of epigenetic regulation
- Discuss the role of transcription factors in gene regulation
- Understand RNA splicing and explain its role in regulating gene expression
- Describe the importance of RNA stability in gene regulation
Eukaryotic Epigenetic Gene Regulation
The human genome encodes over 20,000 genes; each of the 23 pairs of human chromosomes encodes thousands of genes. The DNA in the nucleus is precisely wound, folded, and compacted into chromosomes so that it will fit into the nucleus. It is also organized so that specific segments can be accessed as needed by a specific cell type.
The first level of organization, or packing, is the winding of DNA strands around histone proteins. Histones package and order DNA into structural units called nucleosome complexes, which can control the access of proteins to the DNA regions (Figure 1a). Under the electron microscope, this winding of DNA around histone proteins to form nucleosomes looks like small beads on a string (Figure 1b). These beads (histone proteins) can move along the string (DNA) and change the structure of the molecule.
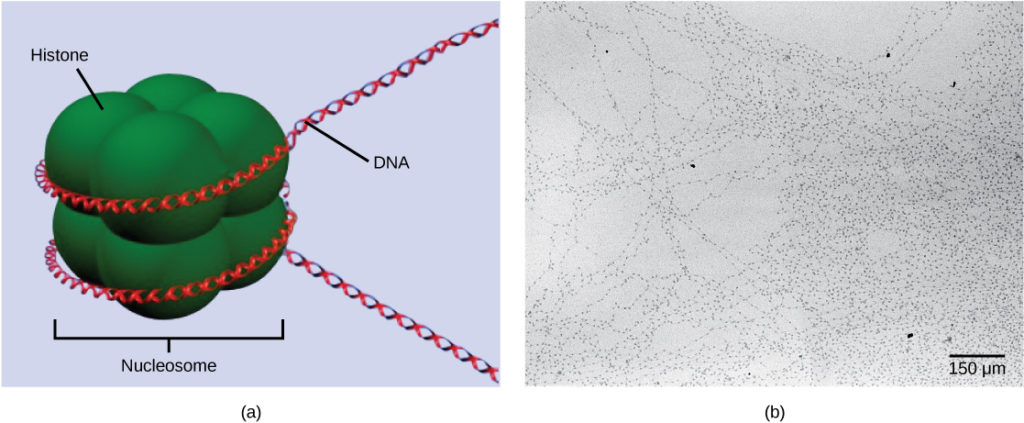
Figure 1. DNA is folded around histone proteins to create (a) nucleosome complexes. These nucleosomes control the access of proteins to the underlying DNA. When viewed through an electron microscope (b), the nucleosomes look like beads on a string. (credit “micrograph”: modification of work by Chris Woodcock)
If DNA encoding a specific gene is to be transcribed into RNA, the nucleosomes surrounding that region of DNA can slide down the DNA to open that specific chromosomal region and allow for the transcriptional machinery (RNA polymerase) to initiate transcription (Figure 2). Nucleosomes can move to open the chromosome structure to expose a segment of DNA, but do so in a very controlled manner.
Practice Question
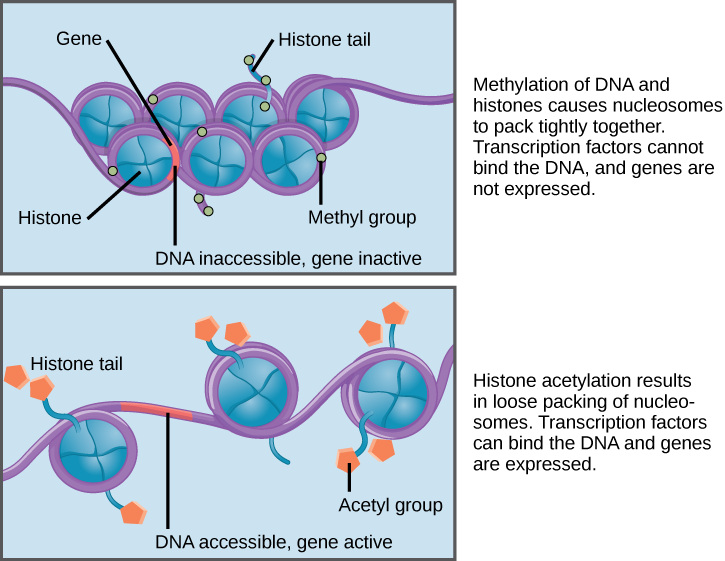
Figure 2. Nucleosomes can slide along DNA. When nucleosomes are spaced closely together (top), transcription factors cannot bind and gene expression is turned off. When the nucleosomes are spaced far apart (bottom), the DNA is exposed. Transcription factors can bind, allowing gene expression to occur. Modifications to the histones and DNA affect nucleosome spacing.
In females, one of the two X chromosomes is inactivated during embryonic development because of epigenetic changes to the chromatin. What impact do you think these changes would have on nucleosome packing?
How the histone proteins move is dependent on signals found on both the histone proteins and on the DNA. These signals are tags added to histone proteins and DNA that tell the histones if a chromosomal region should be open or closed (Figure 3 depicts modifications to histone proteins and DNA). These tags are not permanent, but may be added or removed as needed. They are chemical modifications (phosphate, methyl, or acetyl groups) that are attached to specific amino acids in the protein or to the nucleotides of the DNA. The tags do not alter the DNA base sequence, but they do alter how tightly wound the DNA is around the histone proteins. DNA is a negatively charged molecule; therefore, changes in the charge of the histone will change how tightly wound the DNA molecule will be. When unmodified, the histone proteins have a large positive charge; by adding chemical modifications like acetyl groups, the charge becomes less positive.
The DNA molecule itself can also be modified. This occurs within very specific regions called CpG islands. These are stretches with a high frequency of cytosine and guanine dinucleotide DNA pairs (CG) found in the promoter regions of genes. When this configuration exists, the cytosine member of the pair can be methylated (a methyl group is added). This modification changes how the DNA interacts with proteins, including the histone proteins that control access to the region. Highly methylated (hypermethylated) DNA regions with deacetylated histones are tightly coiled and transcriptionally inactive.

Figure 3. Histone proteins and DNA nucleotides can be modified chemically. Modifications affect nucleosome spacing and gene expression. (credit: modification of work by NIH)
This type of gene regulation is called epigenetic regulation. Epigenetic means “around genetics.” The changes that occur to the histone proteins and DNA do not alter the nucleotide sequence and are not permanent. Instead, these changes are temporary (although they often persist through multiple rounds of cell division) and alter the chromosomal structure (open or closed) as needed. A gene can be turned on or off depending upon the location and modifications to the histone proteins and DNA. If a gene is to be transcribed, the histone proteins and DNA are modified surrounding the chromosomal region encoding that gene. This opens the chromosomal region to allow access for RNA polymerase and other proteins, called transcription factors, to bind to the promoter region, located just upstream of the gene, and initiate transcription. If a gene is to remain turned off, or silenced, the histone proteins and DNA have different modifications that signal a closed chromosomal configuration. In this closed configuration, the RNA polymerase and transcription factors do not have access to the DNA and transcription cannot occur (Figure 2).
View this video that describes how epigenetic regulation controls gene expression.
Like prokaryotic cells, the transcription of genes in eukaryotes requires the actions of an RNA polymerase to bind to a sequence upstream of a gene to initiate transcription. However, unlike prokaryotic cells, the eukaryotic RNA polymerase requires other proteins, or transcription factors, to facilitate transcription initiation. Transcription factors are proteins that bind to the promoter sequence and other regulatory sequences to control the transcription of the target gene. RNA polymerase by itself cannot initiate transcription in eukaryotic cells. Transcription factors must bind to the promoter region first and recruit RNA polymerase to the site for transcription to be established.
View the process of transcription—the making of RNA from a DNA template:
The Promoter and the Transcription Machinery
Genes are organized to make the control of gene expression easier. The promoter region is immediately upstream of the coding sequence. This region can be short (only a few nucleotides in length) or quite long (hundreds of nucleotides long). The longer the promoter, the more available space for proteins to bind. This also adds more control to the transcription process. The length of the promoter is gene-specific and can differ dramatically between genes. Consequently, the level of control of gene expression can also differ quite dramatically between genes. The purpose of the promoter is to bind transcription factors that control the initiation of transcription.
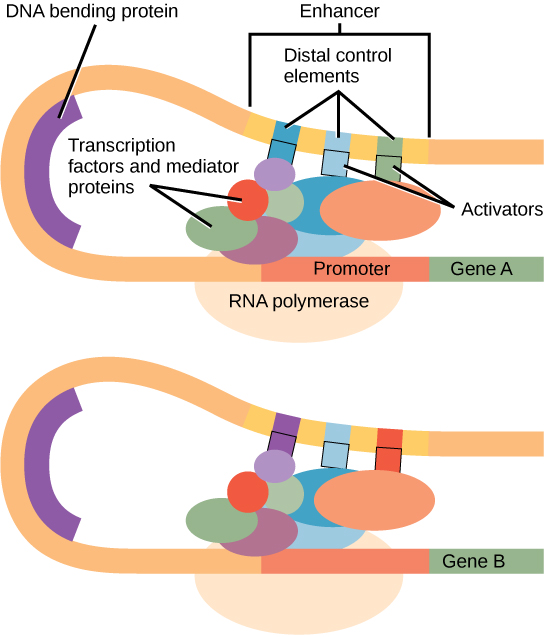
Figure 1. An enhancer is a DNA sequence that promotes transcription. Each enhancer is made up of short DNA sequences called distal control elements. Activators bound to the distal control elements interact with mediator proteins and transcription factors. Two different genes may have the same promoter but different distal control elements, enabling differential gene expression.
Within the promoter region, just upstream of the transcriptional start site, resides the TATA box. This box is simply a repeat of thymine and adenine dinucleotides (literally, TATA repeats). RNA polymerase binds to the transcription initiation complex, allowing transcription to occur. To initiate transcription, a transcription factor (TFIID) is the first to bind to the TATA box. Binding of TFIID recruits other transcription factors, including TFIIB, TFIIE, TFIIF, and TFIIH to the TATA box. Once this complex is assembled, RNA polymerase can bind to its upstream sequence. When bound along with the transcription factors, RNA polymerase is phosphorylated. This releases part of the protein from the DNA to activate the transcription initiation complex and places RNA polymerase in the correct orientation to begin transcription; DNA-bending protein brings the enhancer, which can be quite a distance from the gene, in contact with transcription factors and mediator proteins (Figure 1).
In addition to the general transcription factors, other transcription factors can bind to the promoter to regulate gene transcription. These transcription factors bind to the promoters of a specific set of genes. They are not general transcription factors that bind to every promoter complex, but are recruited to a specific sequence on the promoter of a specific gene. There are hundreds of transcription factors in a cell that each bind specifically to a particular DNA sequence motif. When transcription factors bind to the promoter just upstream of the encoded gene, it is referred to as a cis-acting element, because it is on the same chromosome just next to the gene. The region that a particular transcription factor binds to is called the transcription factor binding site. Transcription factors respond to environmental stimuli that cause the proteins to find their binding sites and initiate transcription of the gene that is needed.
Enhancers and Transcription
In some eukaryotic genes, there are regions that help increase or enhance transcription. These regions, called enhancers, are not necessarily close to the genes they enhance. They can be located upstream of a gene, within the coding region of the gene, downstream of a gene, or may be thousands of nucleotides away.
Enhancer regions are binding sequences, or sites, for transcription factors. When a DNA-bending protein binds, the shape of the DNA changes (Figure 1). This shape change allows for the interaction of the activators bound to the enhancers with the transcription factors bound to the promoter region and the RNA polymerase. Whereas DNA is generally depicted as a straight line in two dimensions, it is actually a three-dimensional object. Therefore, a nucleotide sequence thousands of nucleotides away can fold over and interact with a specific promoter.
Turning Genes Off: Transcriptional Repressors
Like prokaryotic cells, eukaryotic cells also have mechanisms to prevent transcription. Transcriptional repressors can bind to promoter or enhancer regions and block transcription. Like the transcriptional activators, repressors respond to external stimuli to prevent the binding of activating transcription factors.
Practice Questions
The binding of ________ is required for transcription to start.
- a protein
- DNA polymerase
- RNA polymerase
- a transcription factor
What will result from the binding of a transcription factor to an enhancer region?
- decreased transcription of an adjacent gene
- increased transcription of a distant gene
- alteration of the translation of an adjacent gene
- initiation of the recruitment of RNA polymerase
A mutation within the promoter region can alter transcription of a gene. Describe how this can happen.
What could happen if a cell had too much of an activating transcription factor present?
Post-Translational Control of Gene Expression
RNA is transcribed, but must be processed into a mature form before translation can begin. This processing after an RNA molecule has been transcribed, but before it is translated into a protein, is called post-transcriptional modification. As with the epigenetic and transcriptional stages of processing, this post-transcriptional step can also be regulated to control gene expression in the cell. If the RNA is not processed, shuttled, or translated, then no protein will be synthesized.
RNA splicing, the first stage of post-transcriptional control
In eukaryotic cells, the RNA transcript often contains regions, called introns, that are removed prior to translation. The regions of RNA that code for protein are called exons (Figure 1). After an RNA molecule has been transcribed, but prior to its departure from the nucleus to be translated, the RNA is processed and the introns are removed by splicing.
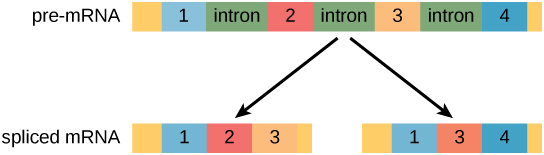
Figure 1. Pre-mRNA can be alternatively spliced to create different proteins.
Alternative RNA Splicing
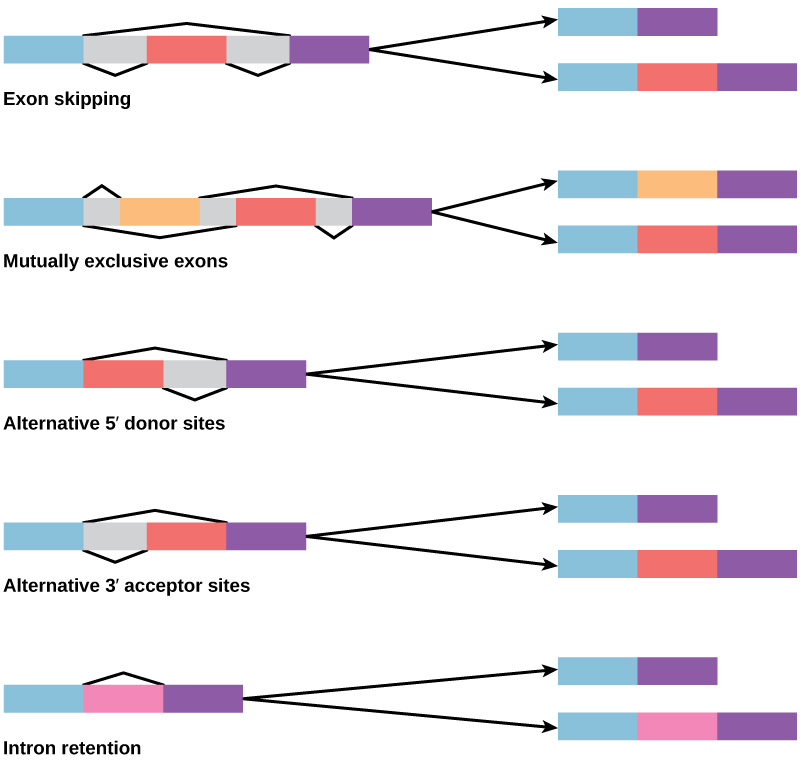
Figure 2. There are five basic modes of alternative splicing.
In the 1970s, genes were first observed that exhibited alternative RNA splicing. Alternative RNA splicing is a mechanism that allows different protein products to be produced from one gene when different combinations of introns, and sometimes exons, are removed from the transcript (Figure 2). This alternative splicing can be haphazard, but more often it is controlled and acts as a mechanism of gene regulation, with the frequency of different splicing alternatives controlled by the cell as a way to control the production of different protein products in different cells or at different stages of development. Alternative splicing is now understood to be a common mechanism of gene regulation in eukaryotes; according to one estimate, 70 percent of genes in humans are expressed as multiple proteins through alternative splicing.
How could alternative splicing evolve? Introns have a beginning and ending recognition sequence; it is easy to imagine the failure of the splicing mechanism to identify the end of an intron and instead find the end of the next intron, thus removing two introns and the intervening exon. In fact, there are mechanisms in place to prevent such intron skipping, but mutations are likely to lead to their failure. Such “mistakes” would more than likely produce a nonfunctional protein. Indeed, the cause of many genetic diseases is alternative splicing rather than mutations in a sequence. However, alternative splicing would create a protein variant without the loss of the original protein, opening up possibilities for adaptation of the new variant to new functions. Gene duplication has played an important role in the evolution of new functions in a similar way by providing genes that may evolve without eliminating the original, functional protein.
Visualize how mRNA splicing happens by watching the process in action in this video:
Control of RNA Stability
Before the mRNA leaves the nucleus, it is given two protective “caps” that prevent the end of the strand from degrading during its journey. The 5′ cap, which is placed on the 5′ end of the mRNA, is usually composed of a methylated guanosine triphosphate molecule (GTP). The poly-A tail, which is attached to the 3′ end, is usually composed of a series of adenine nucleotides. Once the RNA is transported to the cytoplasm, the length of time that the RNA resides there can be controlled. Each RNA molecule has a defined lifespan and decays at a specific rate. This rate of decay can influence how much protein is in the cell. If the decay rate is increased, the RNA will not exist in the cytoplasm as long, shortening the time for translation to occur. Conversely, if the rate of decay is decreased, the RNA molecule will reside in the cytoplasm longer and more protein can be translated. This rate of decay is referred to as the RNA stability. If the RNA is stable, it will be detected for longer periods of time in the cytoplasm.
Binding of proteins to the RNA can influence its stability. Proteins, called RNA-binding proteins, or RBPs, can bind to the regions of the RNA just upstream or downstream of the protein-coding region. These regions in the RNA that are not translated into protein are called the untranslated regions, or UTRs. They are not introns (those have been removed in the nucleus). Rather, these are regions that regulate mRNA localization, stability, and protein translation. The region just before the protein-coding region is called the 5′ UTR, whereas the region after the coding region is called the 3′ UTR (Figure 3). The binding of RBPs to these regions can increase or decrease the stability of an RNA molecule, depending on the specific RBP that binds.

Figure 3. The protein-coding region of mRNA is flanked by 5′ and 3′ untranslated regions (UTRs). The presence of RNA-binding proteins at the 5′ or 3′ UTR influences the stability of the RNA molecule.
RNA Stability and microRNAs
In addition to RBPs that bind to and control (increase or decrease) RNA stability, other elements called microRNAs can bind to the RNA molecule. These microRNAs, or miRNAs, are short RNA molecules that are only 21–24 nucleotides in length. The miRNAs are made in the nucleus as longer pre-miRNAs. These pre-miRNAs are chopped into mature miRNAs by a protein called dicer. Like transcription factors and RBPs, mature miRNAs recognize a specific sequence and bind to the RNA; however, miRNAs also associate with a ribonucleoprotein complex called the RNA-induced silencing complex (RISC). RISC binds along with the miRNA to degrade the target mRNA. Together, miRNAs and the RISC complex rapidly destroy the RNA molecule.
Practice Questions
Which of the following are involved in post-transcriptional control?
- control of RNA splicing
- control of RNA shuttling
- control of RNA stability
- all of the above
Binding of an RNA binding protein will ________ the stability of the RNA molecule.
- increase
- decrease
- neither increase nor decrease
- either increase or decrease
Describe how RBPs can prevent miRNAs from degrading an RNA molecule.
How can external stimuli alter post-transcriptional control of gene expression?
In Summary: Eukaryotic Epigenetic Gene Regulation
In eukaryotic cells, the first stage of gene expression control occurs at the epigenetic level. Epigenetic mechanisms control access to the chromosomal region to allow genes to be turned on or off. These mechanisms control how DNA is packed into the nucleus by regulating how tightly the DNA is wound around histone proteins. The addition or removal of chemical modifications (or flags) to histone proteins or DNA signals to the cell to open or close a chromosomal region. Therefore, eukaryotic cells can control whether a gene is expressed by controlling accessibility to transcription factors and the binding of RNA polymerase to initiate transcription.
To start transcription, general transcription factors, such as TFIID, TFIIH, and others, must first bind to the TATA box and recruit RNA polymerase to that location. The binding of additional regulatory transcription factors to cis-acting elements will either increase or prevent transcription. In addition to promoter sequences, enhancer regions help augment transcription. Enhancers can be upstream, downstream, within a gene itself, or on other chromosomes. Transcription factors bind to enhancer regions to increase or prevent transcription.
Post-transcriptional control can occur at any stage after transcription, including RNA splicing, nuclear shuttling, and RNA stability. Once RNA is transcribed, it must be processed to create a mature RNA that is ready to be translated. This involves the removal of introns that do not code for protein. Spliceosomes bind to the signals that mark the exon/intron border to remove the introns and ligate the exons together. Once this occurs, the RNA is mature and can be translated. RNA is created and spliced in the nucleus, but needs to be transported to the cytoplasm to be translated. RNA is transported to the cytoplasm through the nuclear pore complex. Once the RNA is in the cytoplasm, the length of time it resides there before being degraded, called RNA stability, can also be altered to control the overall amount of protein that is synthesized. The RNA stability can be increased, leading to longer residency time in the cytoplasm, or decreased, leading to shortened time and less protein synthesis. RNA stability is controlled by RNA-binding proteins (RPBs) and microRNAs (miRNAs). These RPBs and miRNAs bind to the 5′ UTR or the 3′ UTR of the RNA to increase or decrease RNA stability. Depending on the RBP, the stability can be increased or decreased significantly; however, miRNAs always decrease stability and promote decay.
Check Your Understanding
Answer the question(s) below to see how well you understand the topics covered in the previous section. This short quiz does not count toward your grade in the class, and you can retake it an unlimited number of times.
Use this quiz to check your understanding and decide whether to (1) study the previous section further or (2) move on to the next section.
Candela Citations
- Introduction to Eukaryotic Gene Regulation. Authored by: Shelli Carter and Lumen Learning. Provided by: Lumen Learning. License: CC BY: Attribution
- Biology. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.8. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.8