20.3.1. A digression: Formation and properties of organometallic reagents
The alkali metals (Li, Na, K etc.) and the alkaline earth metals (Mg and Ca, together with Zn) are good reducing agents, the former being stronger than the latter. These same metals reduce the carbon-halogen bonds of alkyl halides. The halogen is converted to a halide anion, and the carbon bonds to the metal which has characteristics similar to a carbanion (R:-).
Formation of organometallic reagents
Many organometallic reagents are commercially available, however, it is often necessary to make then. The following equations illustrate these reactions for the commonly used metals lithium and magnesium (R may be hydrogen or alkyl groups in any combination).
-
An alkyllithium reagent
R3C−X+2Li→R3C−Li+LiX
-
A Grignard reagent
R3C−X+Mg→R3C−MgX
Halide reactivity in these reactions increases in the order: Cl < Br < I and fluorides are usually not used. The alkyl magnesium halides described in the second reaction are called Grignard reagents after the French chemist, Victor Grignard, who discovered them and received the Nobel prize in 1912 for this work. The other metals mentioned above react in a similar manner, but Grignard and Alkyllithium reagents are the most widely used. Although the formulae drawn here for the alkyllithium and Grignard reagents reflect the stoichiometry of the reactions and are widely used in the chemical literature, they do not accurately depict the structural nature of these remarkable substances. Mixtures of polymeric and other associated and complexed species are in equilibrium under the conditions normally used for their preparation.
A suitable solvent must be used. For alkyllithium formation pentane or hexane is usually used. Diethyl ether can also be used but the subsequent alkyllithium reagent must be used immediately after preparation due to an interaction with the solvent. Ethyl ether or THF are essential for Grignard reagent formation. Lone pair electrons from two ether molecules form a complex with the magnesium in the Grignard reagent (As pictured below). This complex helps stabilize the organometallic and increases its ability to react.

These reactions are obviously substitution reactions, but they cannot be classified as nucleophilic substitutions, as were the earlier reactions of alkyl halides. Because the functional carbon atom has been reduced, the polarity of the resulting functional group is inverted (an originally electrophilic carbon becomes nucleophilic). This change, shown below, makes alkyl lithium and Grignard reagents excellent nucleophiles and useful reactants in synthesis.

Examples


Common organometallic reagents


Organometallic reagents as bases
These reagents are very strong bases (pKa’s of saturated hydrocarbons range are around 50). Although not usually done with Grignard reagents, organolithium reagents can be used as strong bases. Both Grignard reagents and organolithium reagents react with water to form the corresponding hydrocarbon. This is why so much care is needed to insure dry glassware and solvents when working with organometallic reagents.

In fact, the reaction of Grignard reagents and organolithium reagents with water can be exploited to convert alkyl halides into the corresponding hydrocarbon (illustrated below). The halogen is converted to an organometallic reagent and then subsequently reacted with water to form an alkane.

Limitation of organometallic reagents
As discussed above, Grignard and organolithium reagents are powerful bases. Because of this they cannot be used as nucleophiles on compounds which contain acidic hydrogens. If they are used they will act as a base and deprotonate the acidic hydrogen rather than act as a nucleophile and attack the carbonyl. A partial list of functional groups which cannot be used are: alcohols, amides, 1o amines (RNH2), 2o amines (R2NH), carboxylic acids, and terminal alkynes.

Video

20.3.2. Reaction of organometallic reagents with polar bonds
Both ‘Grignard reagents’ and organolithiums are particularly useful in nucleophilic addition reactions with carbonyl electrophiles, such as ketones:

The nucleophilic carbon need not be primary as in the picture above. The synthesis of 2-phenyl-2-propanol, for example, can be carried by reacting phenylmagnesium bromide with acetone.

Addition to formaldehyde gives 1o alcohols

Addition to aldehydes gives 2o alcohols

Addition to ketones gives 3o alcohols

Reaction of organometallic reagents with carbon dioxide: Synthesis of carboxylic acids
Grignard reagents can be also be carboxylated simply by pouring the organic solution over dry ice (solid CO2), then adding aqueous HCl:

Or in general:

Examples

Going from reactants to products simplified

Mechanism for the addition to carbonyls
The mechanism for a Grignard agent is shown. The mechanism for an organolithium reagent is essentially the same.
1) Nucleophilic attack

2) Protonation

Problems
1) Please write the product of the following reactions.

2) Please indicate the starting material required to produce the product.

3) Please give a detailed mechanism and the final product of this reaction

4) Please show two sets of reactants which could be used to synthesize the following molecule using a Grignard reaction.

Answers
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
Reaction with esters
Esters and acid chlorides will react with two molar equivalents of Grignard reagent:

The first reaction is an acyl substitution reaction, and the second is a nucleophilic carbonyl addition. Because ketones and aldehydes are better electrophiles than carboxylic acid derivatives, it is not possible to stop these reactions at the ketone/aldehyde stage.
In general, we see the attachment of two identical R’ groups from the organometallic in the alcohol product:

Carboxylic acids and amides will simply protonate the Grignard reagent, not a terribly productive reaction.
Examples

Exercises
1.
If allylmagnesium chloride (CH2=CH=CH2MgCl) were added to a solution of the following compound and then worked-up with acid, the product would contain a chiral center. Would the product be a racemic mixture (optically inactive) or an enantiomerically pure product (optically active)? Draw both enantiomers.

2.
What combination of carbonyl compound and Grignard (use MgBr) reagent would yield the following alcohols (after workup)?
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Solutions
1.
The result would be a racemic mixture of the following.

2.
(a)  (b)
(b)  (c)
(c) 
(d) 
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
Reaction of organometallics with epoxides
Grignard and organolithium reagents will also react with epoxides, attacking the less hindered carbon (as expected for basic/nucleophilic ring-opening conditions – see section 9.6).

Being a strong nucleophile, the organometallic attacks the epoxide from the less hindered end of the epoxide ring. Note that in the product, the new C-C bond is made to the carbon neighboring the alcohol carbon. (In contrast, when organometallics add to carbonyls, the new C-C bond ends up directly bonded to the alcohol carbon.)
Reaction of nitriles (RCN) with Grignard reagents: Synthesis of ketones
General reaction

Example

Mechanism
1) Nucleophilic attack by the Grignard reagent

2) Protonation

3) Protonation

4) Nucleophilic attack by water

5) Proton Transfer

6) Leaving group removal

7) Deprotonation
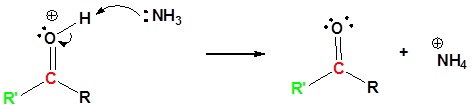
Contributors
Prof. Steven Farmer (Sonoma State University)
20.3.3. Terminal alkynes as carbon nucleophiles
Recall from section 9.8 that the hydrogen on a terminal alkyne is somewhat acidic, with a pKa of approximately 25. This means that, given a strong enough base, terminal alkyne can be deprotonated, yielding a powerful carbanion nucleophile, which we used in SN2 reactions. Sodium hydride, or sodium amide in liquid ammonia is often used for this purpose.

The alkynyl carbanion can then be combined with a suitable electrophile, such as a primary alkyl bromide, in a carbon-carbon bond-forming SN2 displacement reaction. However it can also add to polar bonds in the same way as a Grignard reagent, for example giving an alcohol when added to a ketone.
Grignard reagents will not react efficiently in SN2 reactions with alkyl halides (the Gilman reagent, described below, can be used for this purpose).
Contributors
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
20.3.4. Reactions of organocuprates (Gilman Reagents)
Learning Objectives
After completing this section, you should be able to
- write an equation for the formation of an alkyllithium from an alkyl halide.
- write an equation for the formation of a lithium dialkylcopper (Gilman) reagent from an alkyllithium and copper(I) iodide.
- write an equation for the coupling of a lithium dialkylcopper reagent with an alkyl halide (i.e., a Corey-House synthesis).
- draw the structure of the product formed from a given Corey-House synthesis.
- identify the reagents needed to convert two given organohalides to a specified hydrocarbon through a Corey-House synthesis.
Preparation of Gilman reagents
The Gilman reagent is a lithium diorganocopper species that can be prepared from organolithium compounds:

Coupling reactions of Gilman reagents with alkyl and acyl halides
Gilman reagents are useful in that, unlike Grignard reagents, they will efficiently react in SN2 reactions with alkyl halides, even when the halogen is bonded to an sp2-hybridized, alkene carbon (remember from section 8.2C that SN2 reactions typically do not occur at sp2-hybridized carbons!)

In general, we see:

Examples



Exercises
- Starting with alkyl halides containing no more than four carbon atoms, how would you synthesize each of the following alkanes?
- 2,5-dimethylhexane
- 2-methylhexane
Answers
Conjugate addition of Gilman reagents
Another useful reaction of organocuprates is the so-called “conjugate addition” to C=C-C=O compounds, often called . Whereas RMgX and RLi will simply add normally to the C=O of such compounds, the R2CuLi will add to the alkene portion rather than directly to the carbonyl:

Examples
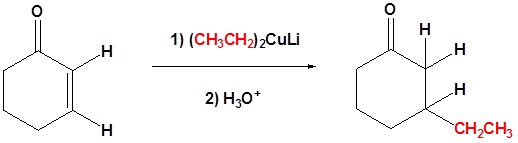
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
Video
https://youtu.be/a-ksnhFbTRs

Candela Citations
- 19.7 Nucleophilic Addition of Grignard Reagents and Hydride Reagents: Alcohol Formation. Authored by: Dr. Dietmar Kennepohl and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_19%3A_Aldehydes_and_Ketones%3A_Nucleophilic_Addition_Reactions/19.07_Nucleophilic_Addition_of_Grignard_Reagents_and_Hydride_Reagents%3A_Alcohol_Formation. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Grignard and Organolithium Reagents. Authored by: Prof. Steven Farmer and William Reusch. Located at: https://chem.libretexts.org/?title=Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Aldehydes_and_Ketones/Synthesis_of_Aldehydes_%26_Ketones/Grignard_and_Organolithium_Reagents. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 10.7: Organometallic Coupling Reactions. Authored by: Dr. Dietmar Kennepohl and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_10%3A_Organohalides/10.07_Organometallic_Coupling_Reactions. Project: Chemistry LibreText. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 17.5 Alcohols from Reaction of Carbonyl Compounds: Grignard Reagents. Authored by: Dr. Dietmar Kennepohl and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_17%3A_Alcohols_and_Phenols/17.05_Alcohols_from_Reaction_of_Carbonyl_Compounds%3A_Grignard_Reagents. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Conversion to ketones using Grignard reagents. Authored by: Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Nitriles/Reactivity_of_Nitriles/Conversion_to_ketones_using_Grignard_reagents. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Organic Chemistry With a Biological Emphasis . Authored by: Tim Soderberg. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/13%3A_Reactions_with_stabilized_carbanion_intermediates_I/13.6%3A_Synthetic_parallel_-_carbon_nucleophiles_in_the_lab#13.6C:_Terminal_alkynes_as_carbon_nucleophiles. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 10.7: Organometallic Coupling Reactions. Authored by: Dr. Dietmar Kennepohl and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_10%3A_Organohalides/10.07_Organometallic_Coupling_Reactions. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Organocuprate reagents convert acid chlorides to ketones. Authored by: Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Acid_Halides/Reactions_of_Acid_Halides/Organocuprate_reagents_convert_acid_chlorides_to_ketones. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike


