Nucleophilic addition of amines: Imine and enamine formation
Objectives
After completing this section, you should be able to
- write equations to describe the reactions that occur between aldehydes or ketones and primary or secondary amines.
- identify the product formed from the reaction of a given aldehyde or ketone with a given primary or secondary amine.
- identify the aldehyde or ketone, the amine, or both, required in the synthesis of a given imine or enamine.
- write the detailed mechanism for the reaction of an aldehyde or ketone with a primary amine.
- write the detailed mechanism for the reaction of an aldehyde or ketone with a secondary amine.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- 2,4‑dinitrophenylhydrazone
- enamine
- imine
Study Notes
An imine is a compound that contains the structural unit

An enamine is a compound that contains the structural unit

Both of these types of compound can be prepared through the reaction of an aldehyde or ketone with an amine.
You may have the opportunity to observe the reaction of an aldehyde and ketone with 2,4‑dinitrophenylhydrazine (Brady’s reagent) to form a 2,4‑dinitrophenylhydrozone in the laboratory. This is a classical organic chemistry test to confirm the presence of a carbonyl group. The reaction produces very colourful and bright precipitates of yellow, orange and red.
If you can understand why the two reactions of imine and enamine formation are essentially identical, and can write a detailed mechanism for each one, you are well on the way to mastering organic chemistry. If you understand how and why these reactions occur, you can keep the amount of material that you need to memorize to a minimum.
Reaction with primary amines to form imines
The reaction of aldehydes and ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff bases (compounds having a C=N function). Water is eliminated in the reaction, which is acid-catalyzed and reversible in the same sense as acetal formation. The pH for reactions which form imine compounds must be carefully controlled. The rate at which these imine compounds are formed is generally greatest near a pH of 5, and drops at higher and lower pH’s. At high pH there will not be enough acid to protonate the OH in the intermediate to allow for removal as H2O. At low pH most of the amine reactant will be tied up as its ammonium conjugate acid and will become non-nucleophilic.

Converting reactants to products simply

Examples of imine forming reactions


Mechanism of imine formation
1) Nucleophilic addition

2) Proton transfer
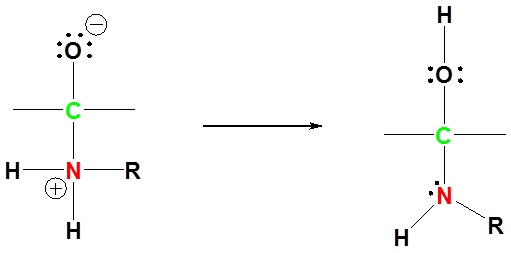
3) Protonation of OH

4) Removal of water (nucleophile elimination)

5) Deprotonation

Reversibility of imine forming reactions
Imines can be hydrolyzed back to the corresponding primary amine under acidic conditions.

Reactions involving other reagents of the type Y-NH2
Imines are sometimes difficult to isolate and purify due to their sensitivity to hydrolysis. Consequently, other reagents of the type Y–NH2 have been studied, and found to give stable products (R2C=N–Y) useful in characterizing the aldehydes and ketones from which they are prepared. Some of these reagents are listed in the following table, together with the structures and names of their carbonyl reaction products. Hydrazones are used as part of the Wolff-Kishner reduction and will be discussed in more detail in section 21.6.

With the exception of unsubstituted hydrazones, these derivatives are easily prepared and are often crystalline solids – even when the parent aldehyde or ketone is a liquid. Since melting points can be determined more quickly and precisely than boiling points, derivatives such as these are useful for comparison and identification of carbonyl compounds. It should be noted that although semicarbazide has two amino groups (–NH2) only one of them is a reactive amine. The other is amide-like and is deactivated by the adjacent carbonyl group.
Exercises
1)Please draw the products of the following reactions.

2) Please draw the structure of the reactant needed to produce the indicated product.

3) Please draw the products of the following reactions.
Answers
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Reaction with secondary amines to form enamines
Most aldehydes and ketones react with 2º-amines to give products known as enamines. It should be noted that, like acetal formation, these are acid-catalyzed reversible reactions in which water is lost. Consequently, enamines are easily converted back to their carbonyl precursors by acid-catalyzed hydrolysis.

Mechanism
1) Nucleophilic addition

2) Proton transfer

3) Protonation of OH

4) Removal of water (nucleophile elimination)

5) Deprotonation

Reversibility of Enamines


Exercises
1) Please draw the products for the following reactions.

2) Please give the structure of the reactant needed to product the following product

Answers
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
Candela Citations
- Imine formation. Authored by: Martin A. Walker. Provided by: SUNY Potsdam. Project: Organic chemistry: An open textbook. License: CC BY-SA: Attribution-ShareAlike
- 19.8 Nucleophilic Addition of Amines: Imine and Enamine Formation. Authored by: Dr. Dietmar Kennepohl and Prof. Steven Farmer . Provided by: Chemistry LibreTexts. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_19%3A_Aldehydes_and_Ketones%3A_Nucleophilic_Addition_Reactions/19.08_Nucleophilic_Addition_of_Amines%3A_Imine_and_Enamine_Formation. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Organic Chemistry With a Biological Emphasis . Authored by: Tim Soderberg. Provided by: Chemistry LibreTexts. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg). License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike





