Alcohols
Alcohols (R-OH) take the suffix “-ol” with an infix numerical bonding position: CH3CH2CH2OH is propan-1-ol. The suffixes -diol, -triol, -tetraol, etc., are used for multiple -OH groups: Ethylene glycol CH2OHCH2OH is ethane-1,2-diol.
If higher precedence functional groups are present (see order of precedence, below), the prefix “hydroxy” is used with the bonding position: CH3CHOHCOOH is 2-hydroxypropanoic acid.
Amines
Amines (R-NH2) are named for the attached alkane chain with the suffix “-amine” (e.g. CH3NH2 methanamine). If necessary, the bonding position is infixed: CH3CH2CH2NH2 propan-1-amine, CH3CHNH2CH3 propan-2-amine. The prefix form is “amino-“.
For secondary amines (of the form R-NH-R), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic N: CH3NHCH2CH3 is N-methylethanamine. Tertiary amines (R-NR-R) are treated similarly: CH3CH2N(CH3)CH2CH2CH3 is N-ethyl-N-methylpropanamine. Again, the substituent groups are ordered alphabetically.
Amides
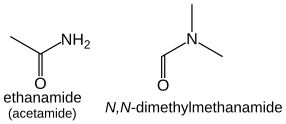
Amides (R-CO-NH2) take the suffix “-amide”, or “-carboxamide” if the carbon in the amide group cannot be included in the main chain. The prefix form is both “carbamoyl-” and “amido-“.
Amides that have additional substituents on the nitrogen are treated similarly to the case of amines: they are ordered alphabetically with the location prefix N: HCON(CH3)2 is N,N-dimethylmethanamide.
Candela Citations
- CHEM 342 XII 7 2 and XII 7 3. Authored by: Martin A. Walker. Provided by: SUNY Potsdam. Located at: https://www.youtube.com/watch?v=5M68UT0pi3s. License: CC BY-SA: Attribution-ShareAlike
- IUPAC nomenclature of organic chemistry. Authored by: Wikipedia contributors. Provided by: Wikimedia Foundation. Located at: https://en.wikipedia.org/w/index.php?title=IUPAC_nomenclature_of_organic_chemistry&oldid=876548225. Project: Wikipedia. License: CC BY-SA: Attribution-ShareAlike