1.1. Introduction to organic chemistry
Learning objectives
- Define organic chemistry.
- Identify organic molecules as alkanes, alkenes, alkynes, alcohols, or carboxylic acids.
Organic chemistry is the study of the chemistry of carbon compounds. Carbon is singled out because it has a chemical diversity unrivaled by any other chemical element. Its diversity is based on the following:
- Carbon atoms bond reasonably strongly with other carbon atoms.
- Carbon atoms bond reasonably strongly with atoms of other elements.
- Carbon atoms make a large number of covalent bonds (four).
Curiously, elemental carbon is not particularly abundant. It does not even appear in the list of the most common elements in Earth’s crust. Nevertheless, all living things consist of organic compounds.
Most organic chemicals are covalent compounds, which is why we introduce organic chemistry here. By convention, compounds containing carbonate ions and bicarbonate ions, as well as carbon dioxide and carbon monoxide, are not considered part of organic chemistry, even though they contain carbon.
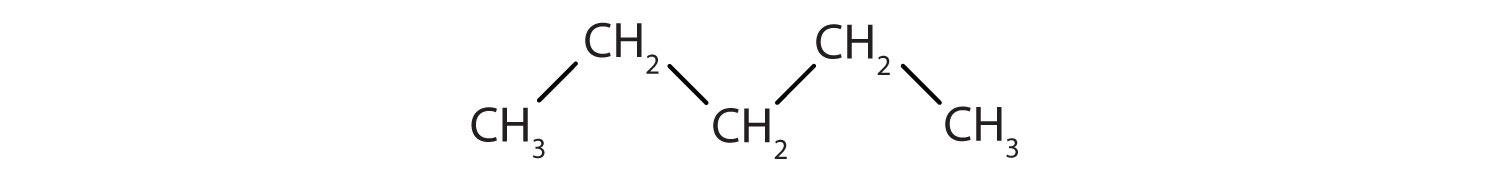
The simplest organic compounds are the hydrocarbons, compounds composed of carbon and hydrogen atoms only. Some hydrocarbons have only single bonds and appear as a chain (which can be a straight chain or can have branches) of carbon atoms also bonded to hydrogen atoms. These hydrocarbons are called alkanes (saturated hydrocarbons). Each alkane has a characteristic, systematic name depending on the number of carbon atoms in the molecule. These names consist of a stem that indicates the number of carbon atoms in the chain plus the ending –ane. The stem meth– means one carbon atom, so methane is an alkane with one carbon atom. Similarly, the stem eth– means two carbon atoms; ethane is an alkane with two carbon atoms. Continuing, the stem prop– means three carbon atoms, so propane is an alkane with three carbon atoms. Figure 1.1. “Formulas and Molecular Models of the Three Simplest Alkanes” gives the formulas and the molecular models of the three simplest alkanes. (For more information about alkanes, see section 3.3.)
Figure 1.1. Formulae and molecular models of the three simplest alkanes
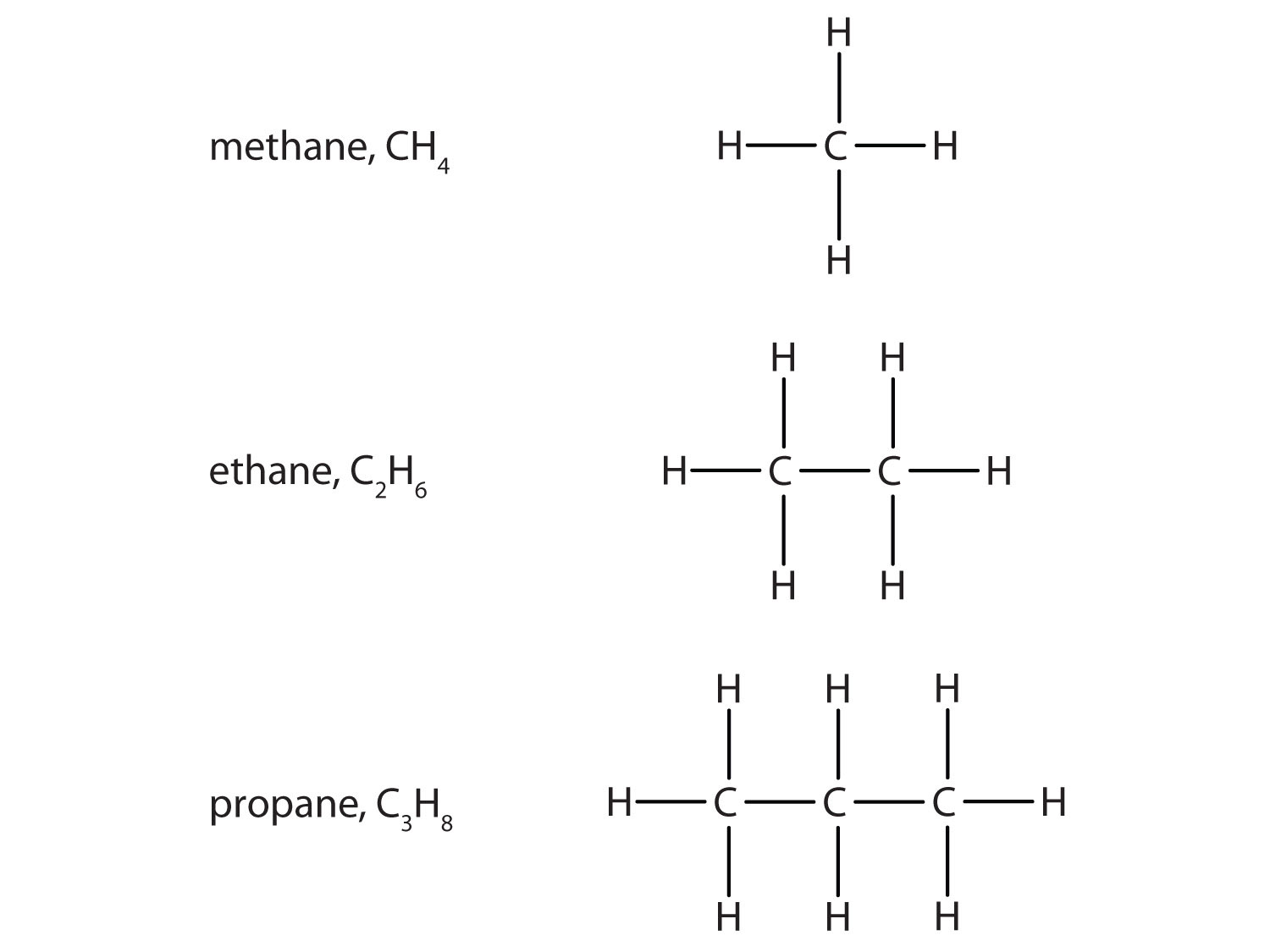
The three smallest alkanes are methane, ethane, and propane.
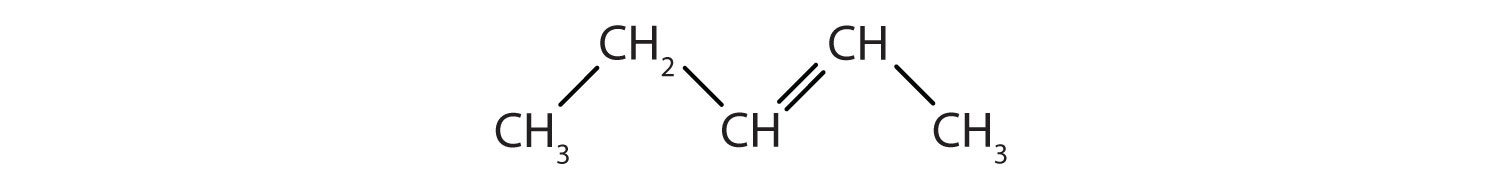
Some hydrocarbons have one or more carbon–carbon double bonds (denoted C=C). These hydrocarbons are called alkenes (see section 3.2. for more information) Note that the names of alkenes have the same stem as the alkane with the same number of carbon atoms in its chain but have the ending –ene. Thus, ethene is an alkene with two carbon atoms per molecule, and propene is a compound with three carbon atoms and one double bond.
Figure 1.2. Formulas and Molecular Models of the Two Simplest Alkenes
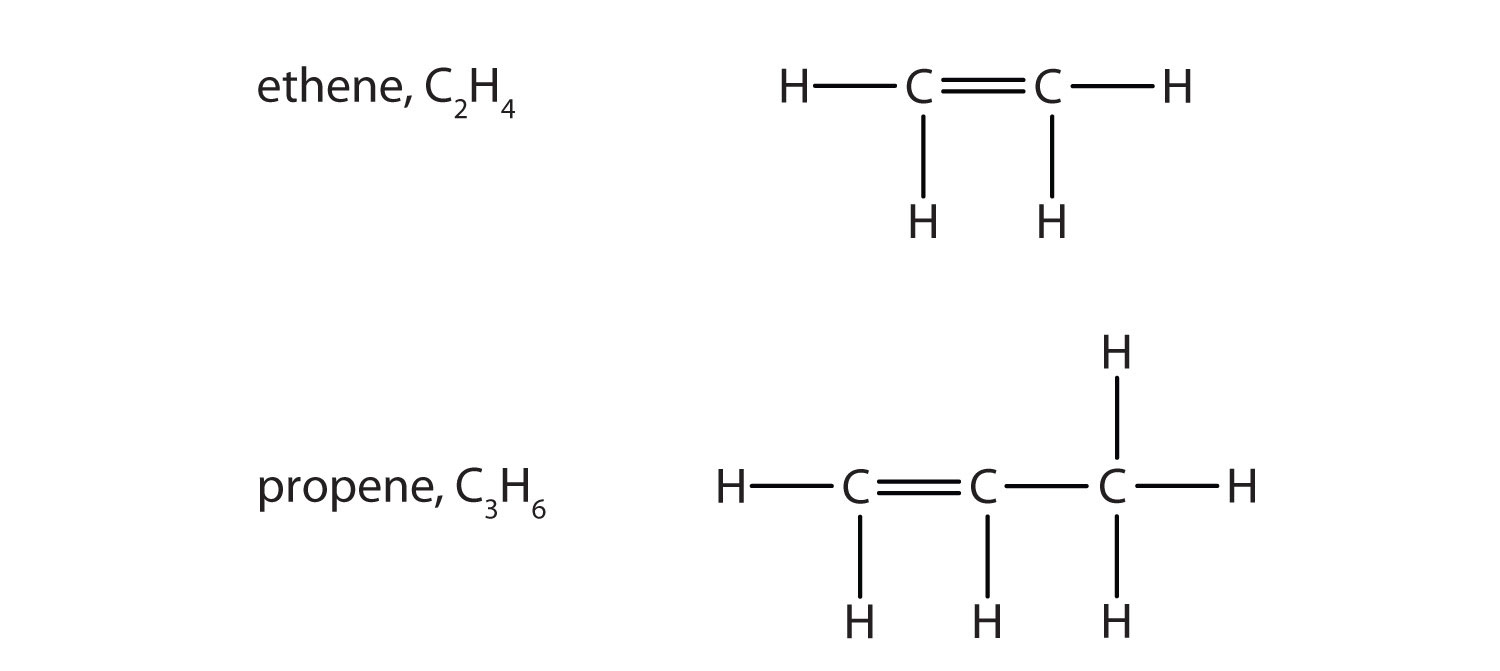
Ethene is commonly called ethylene, while propene is commonly called propylene.
Alkynes are hydrocarbons with a carbon–carbon triple bond (denoted C≡C) as part of their carbon skeleton (see section 3.2. for more information). The names for alkynes have the same stems as for alkanes but with the ending –yne.
Figure 1.3. Formulas and Molecular Models of the Two Simplest Alkynes
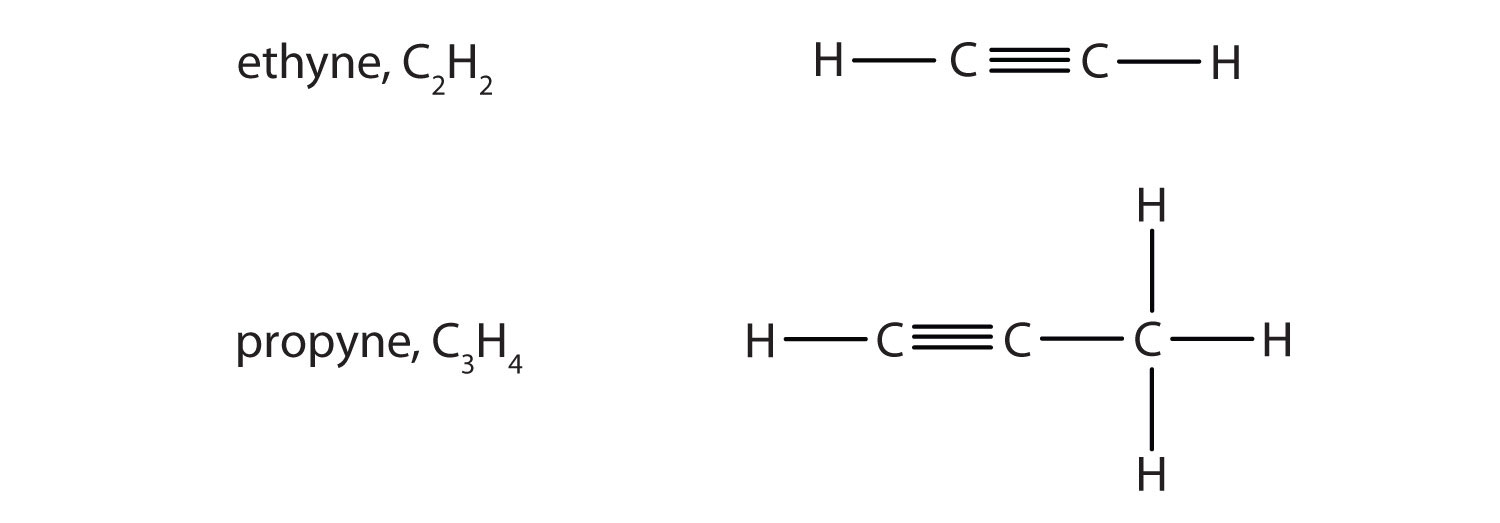
Ethyne is more commonly called acetylene.
To your health: saturated and unsaturated fats
Hydrocarbons are not the only compounds that can have carbon–carbon double bonds. A group of compounds called fats can have them as well, and their presence or absence in the human diet is becoming increasingly correlated with health issues.
Fats are combinations of long-chain organic compounds (fatty acids) and glycerol (C3H8O3). The long carbon chains can have either all single bonds, in which case the fat is classified as saturated, or one or more double bonds, in which case it is a monounsaturated or a polyunsaturated fat, respectively. Saturated fats are typically solids at room temperature; beef fat (tallow) is one example. Mono- or polyunsaturated fats are likely to be liquids at room temperature and are often called oils. Olive oil, flaxseed oil, and many fish oils are mono- or polyunsaturated fats.
Some studies have linked higher amounts of saturated fats in people’s diets with a greater likelihood of developing heart disease, high cholesterol, and other diet-related diseases. In contrast, increases in unsaturated fats (either mono- or polyunsaturated) have been linked to a lower incidence of certain diseases. Thus, there have been recommendations by government bodies and health associations to decrease the proportion of saturated fat and increase the proportion of unsaturated fat in the diet. Most of these organizations also recommend decreasing the total amount of fat in the diet. A difference as simple as the difference between a single and double carbon–carbon bond can have a significant impact on health.
The carbon–carbon double and triple bonds are examples of functional groups in organic chemistry. A functional group is a specific structural arrangement of atoms or bonds that imparts a characteristic chemical reactivity to a molecule. Alkanes have no functional group, and they are mostly inert (unreactive). A carbon–carbon double bond is considered a functional group because carbon–carbon double bonds chemically react in specific ways that differ from reactions of alkanes (for example, under certain circumstances, alkenes react with water); a carbon–carbon triple bond also undergoes certain specific chemical reactions. In the remainder of this section, we introduce two other common functional groups.
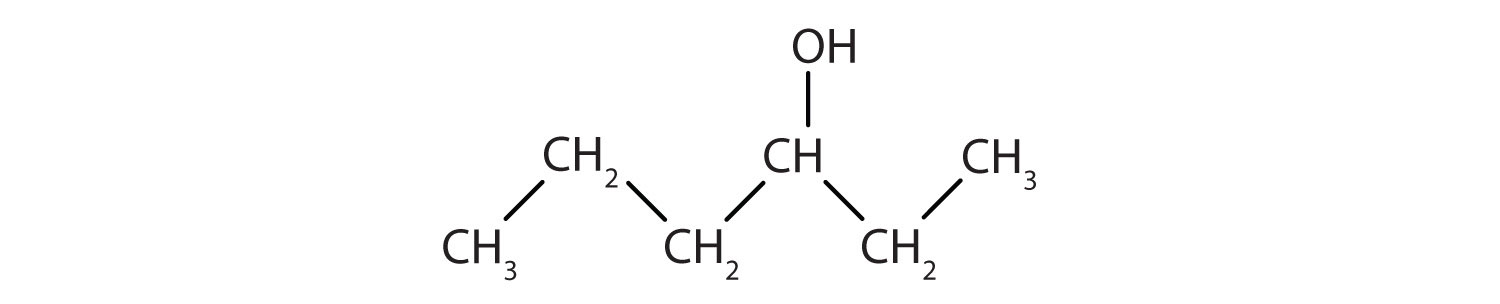
If an OH group (also called a hydroxyl group) is substituted for a hydrogen atom in a hydrocarbon molecule, the compound is an alcohol. Alcohols are named using the parent hydrocarbon name but with the final –e dropped and the suffix –ol attached. The two simplest alcohols are methanol and ethanol (see Figure 1.4.).
Figure 1.4. The two simplest organic alcohol compounds
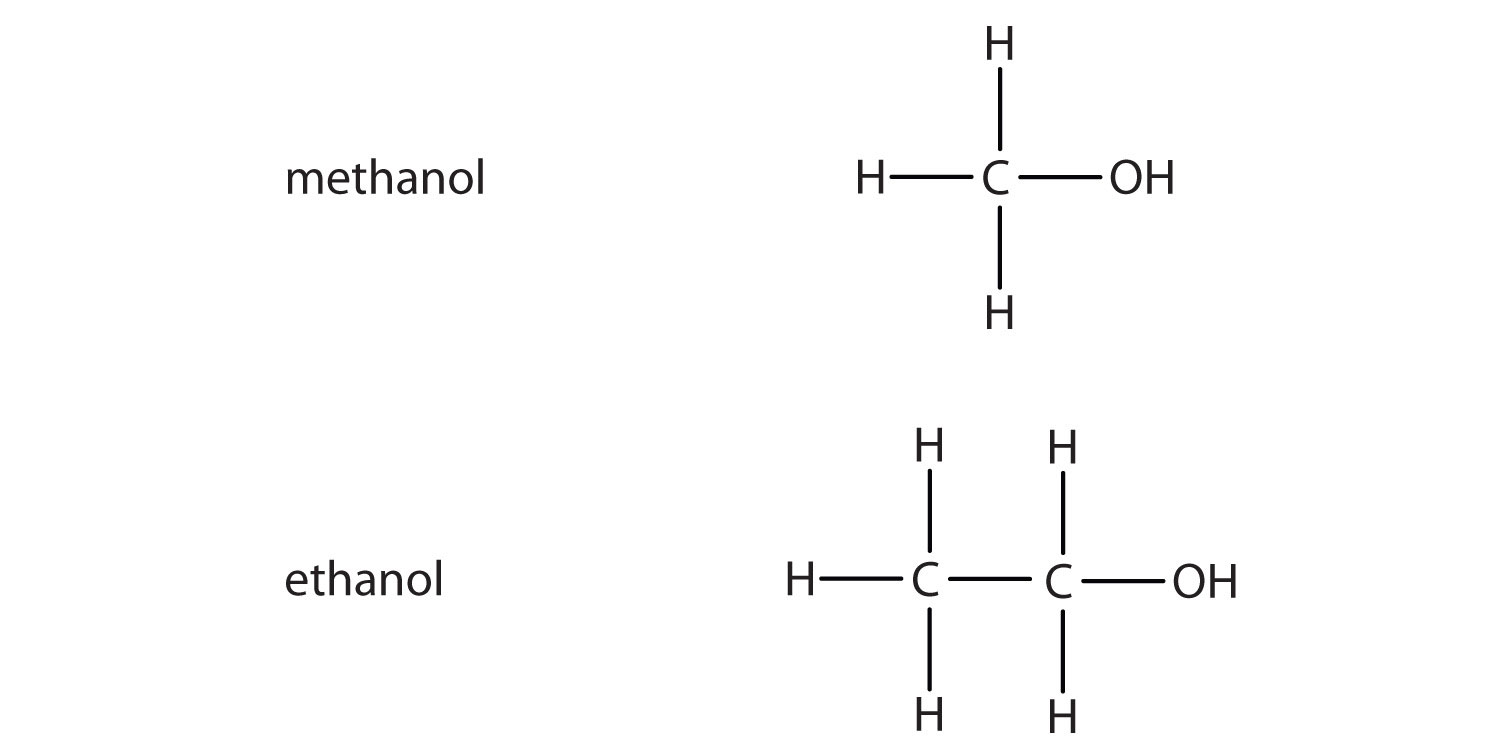
Alcohols have an OH functional group in the molecule. Ethanol (also called ethyl alcohol) is the alcohol in alcoholic beverages. Other alcohols include methanol (or methyl alcohol), which is used as a solvent and a cleaner, and 2-propanol (also called isopropyl alcohol or rubbing alcohol), which is used as a medicinal disinfectant. Neither methanol nor isopropyl alcohol should be ingested, as they are toxic even in small quantities. Cholesterol is an example of a more complex alcohol.
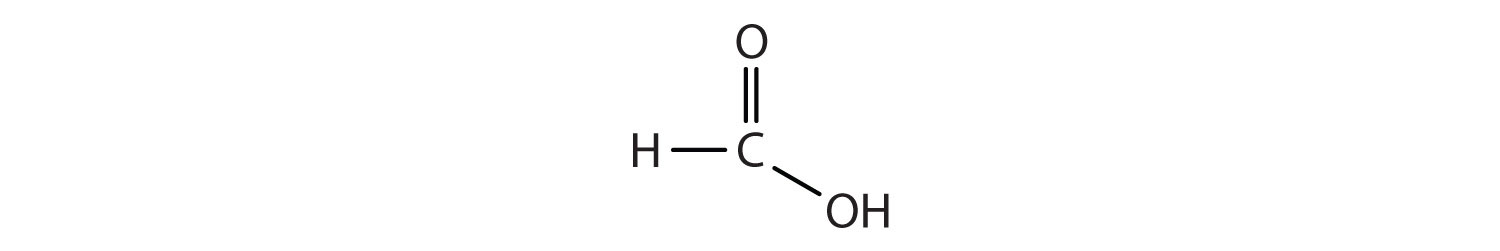
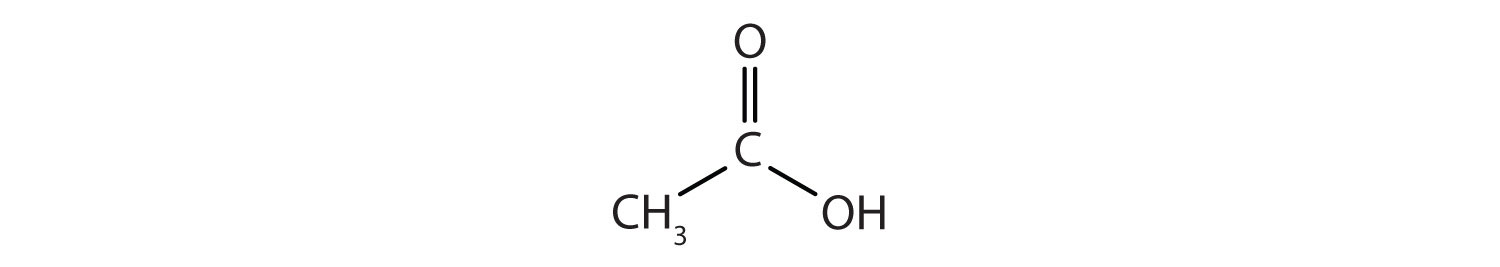
Another important family of organic compounds has a carboxyl group, in which a carbon atom is double-bonded to an oxygen atom and to an OH group. Compounds with a carboxyl functional group are called carboxylic acids, and their names end in –oic acid. The two simplest carboxylic acids are shown in Figure 1.5. They are perhaps best known by the common names formic acid (found in the stingers of ants) and acetic acid (found in vinegar). The carboxyl group is sometimes written in molecules as COOH.
Figure 1.5. The two smallest organic acids
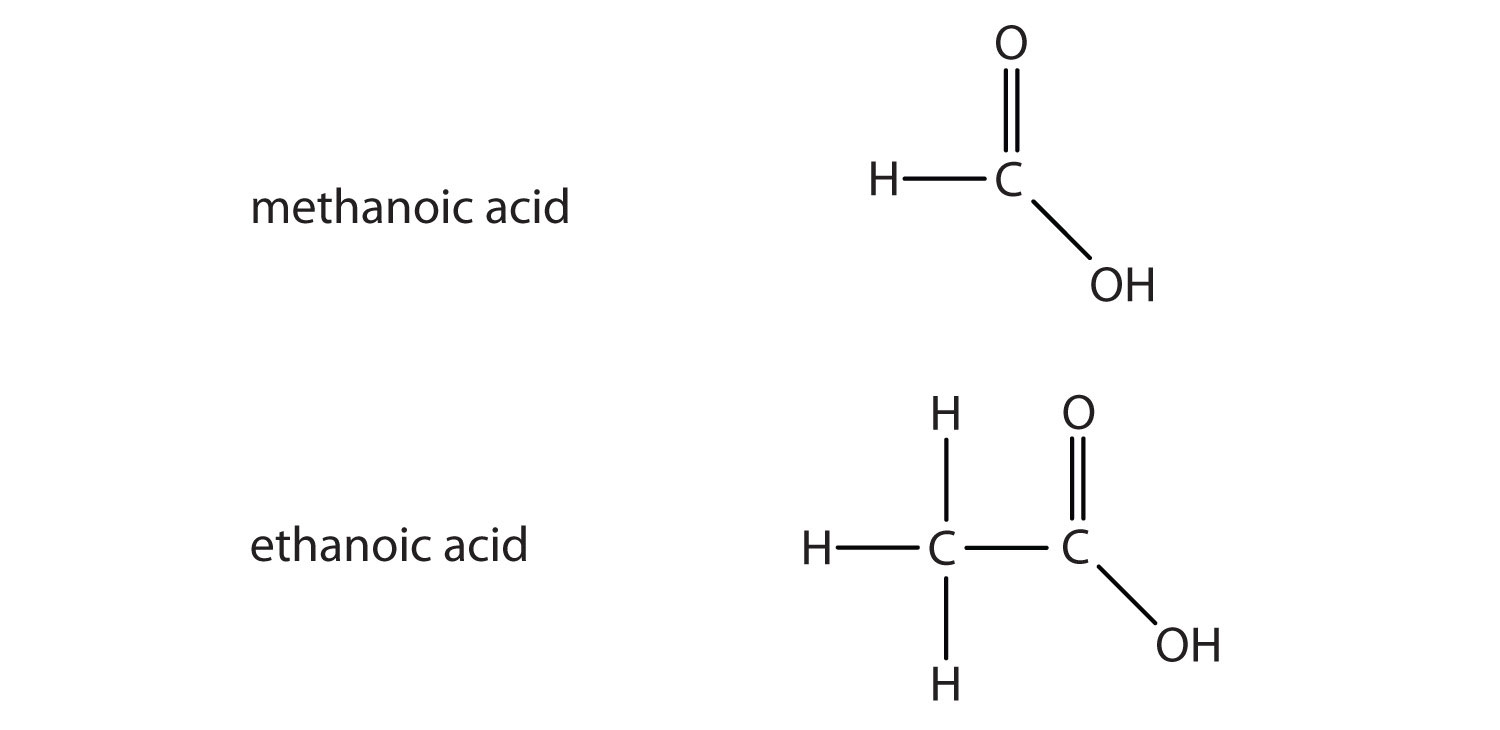
Many organic compounds are considerably more complex than the examples described here. Many compounds contain more than one functional group. The formal names can also be quite complex. In section 1.6. we will examine functional groups in more detail, and we will learn about the system of naming (nomenclature) for hydrocarbons in chapter 3.
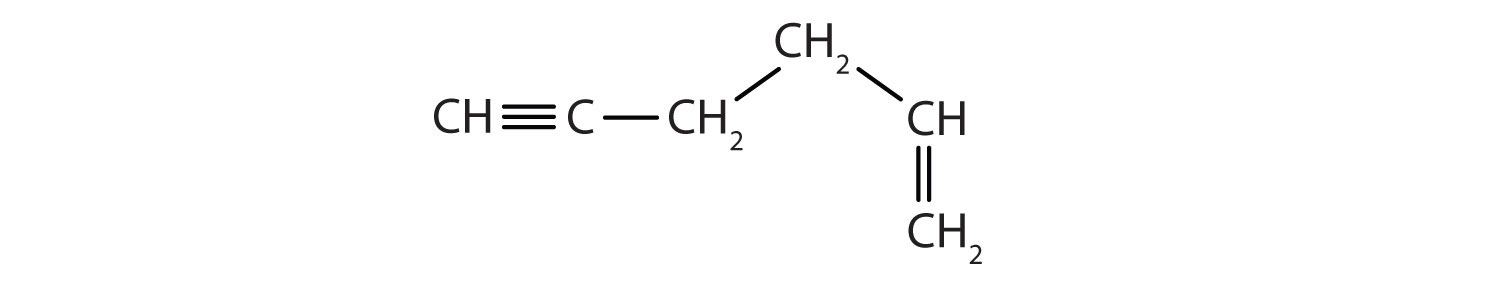
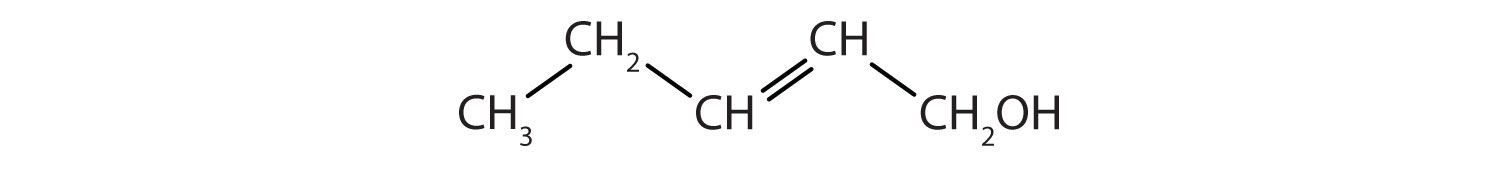
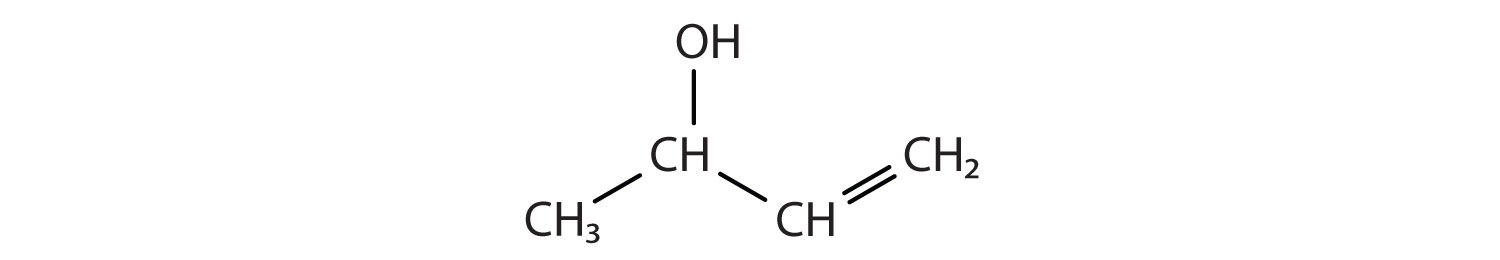
Example 1
Identify the functional group(s) in each molecule as a double bond, a triple bond, an alcohol, or a carboxyl.
- CH3CH2CH2CH2OH
-
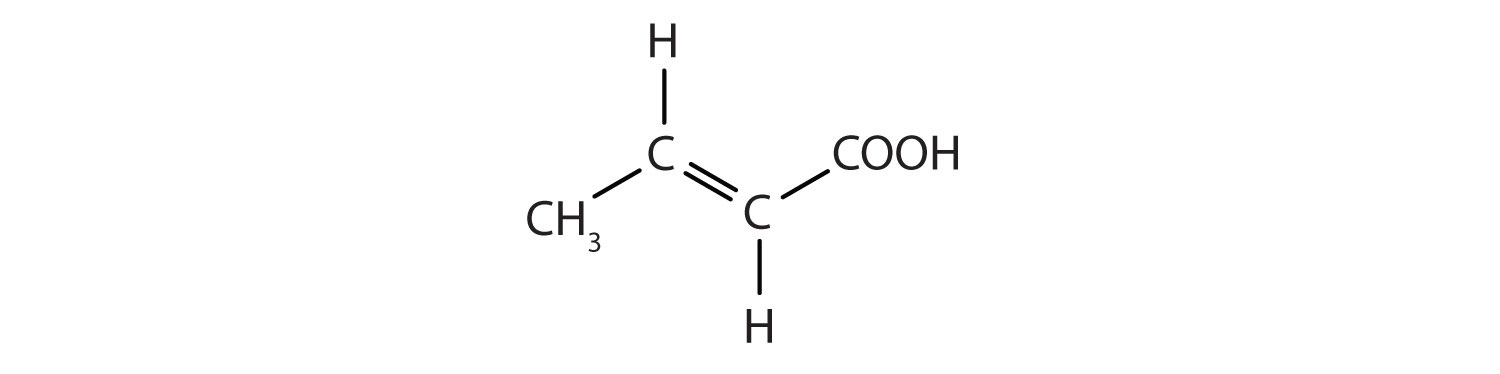
-
-
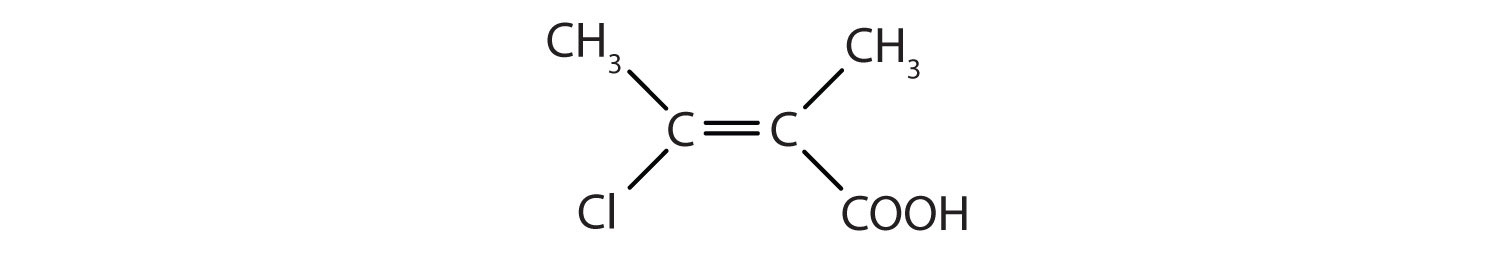
Solution
- This molecule has an alcohol functional group.
- This molecule has a double bond and a carboxyl functional group.
- This molecule has an alcohol functional group.
- This molecule has a double bond and a carboxyl functional group.
Skill-building exercise
Identify the functional group(s) in each molecule as a double bond, a triple bond, an alcohol, or a carboxyl.
Concept review exercises
-
What is organic chemistry?
-
What is a functional group? Give at least two examples of functional groups.
Answers
-
Organic chemistry is the study of the chemistry of carbon compounds.
-
A functional group is a specific structural arrangement of atoms or bonds that imparts a characteristic chemical reactivity to the molecule; alcohol group and carboxylic group (answers will vary).
Key takeaways
- Organic chemistry is the study of the chemistry of carbon compounds.
- Organic molecules can be classified according to the types of elements and bonds in the molecules.
Exercises
-
Give three reasons why carbon is the central element in organic chemistry.
-
Are organic compounds based more on ionic bonding or covalent bonding? Explain.
-
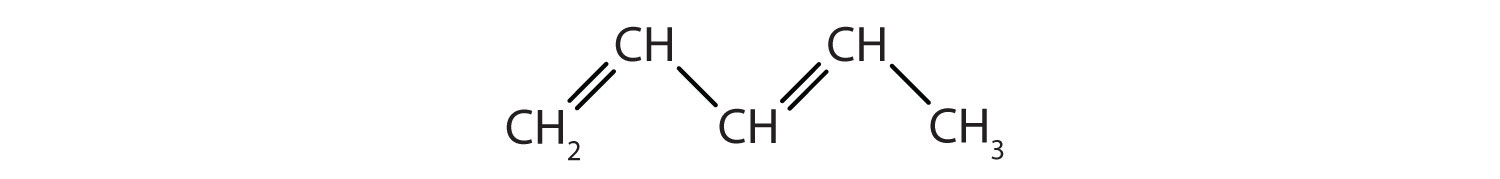
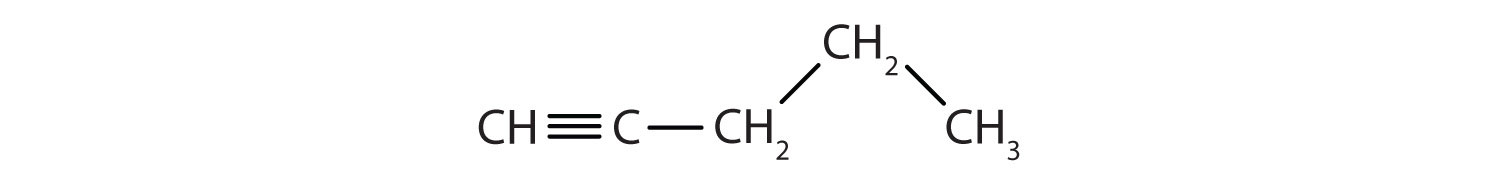
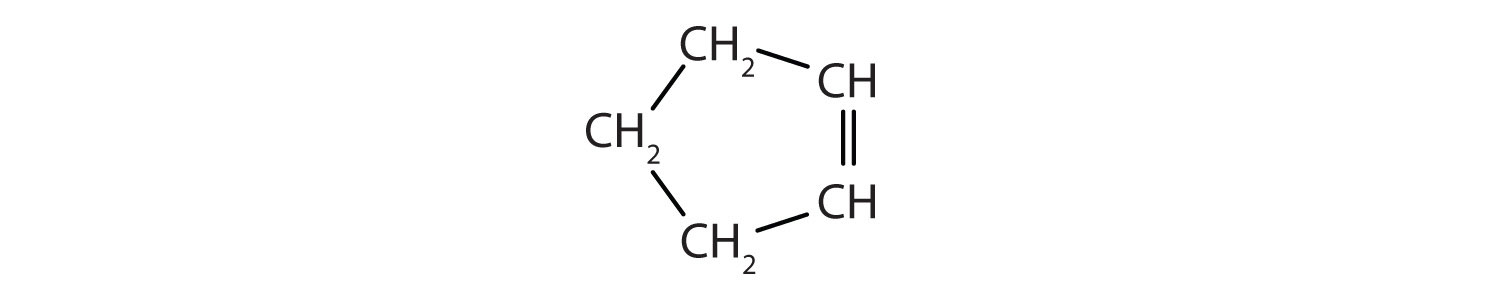
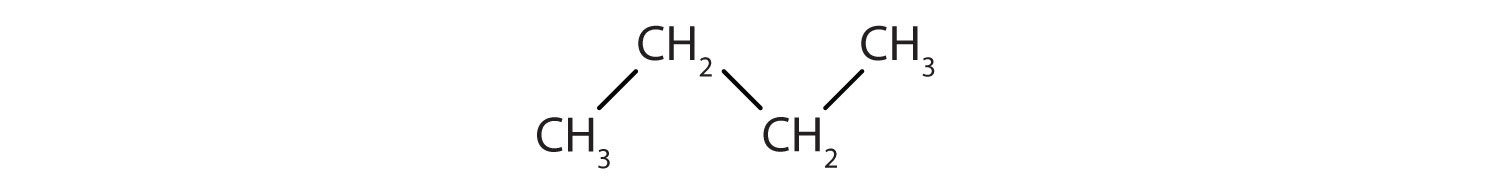
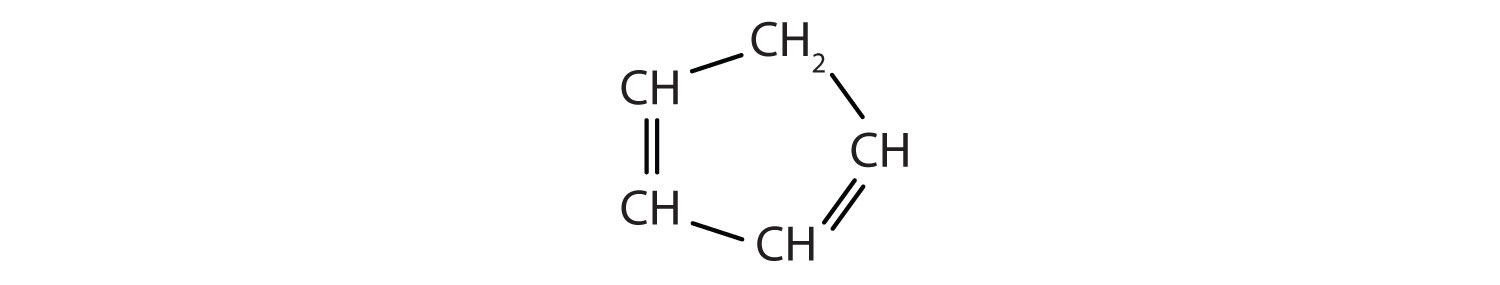
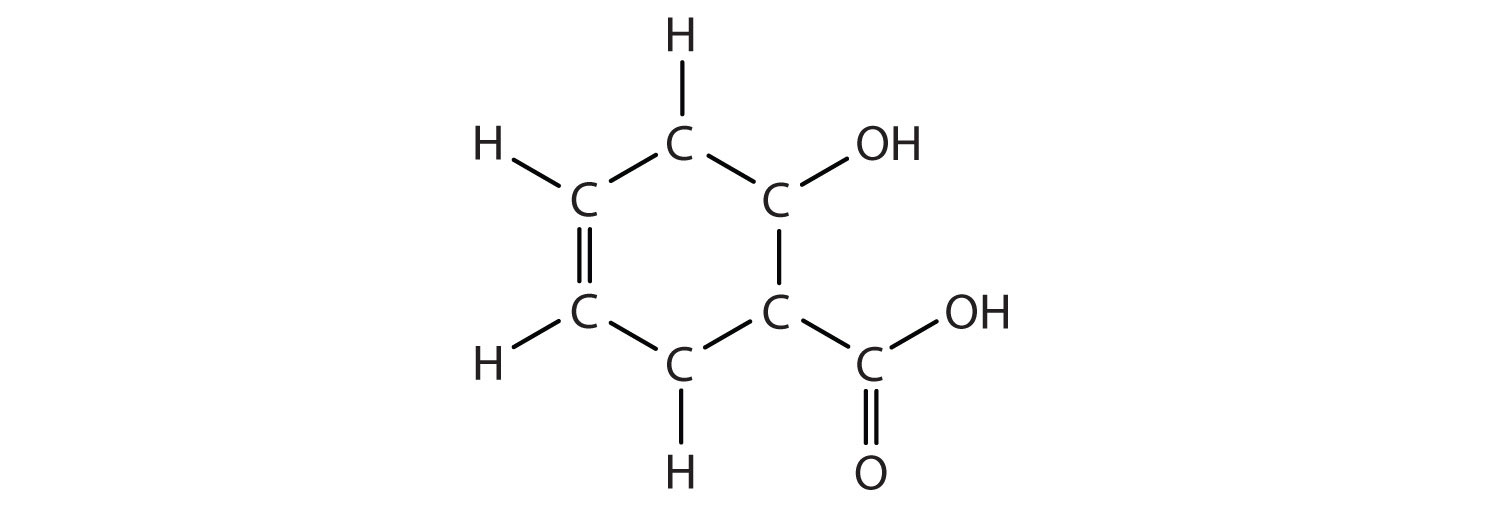
Identify the type of hydrocarbon in each structure.
-
-
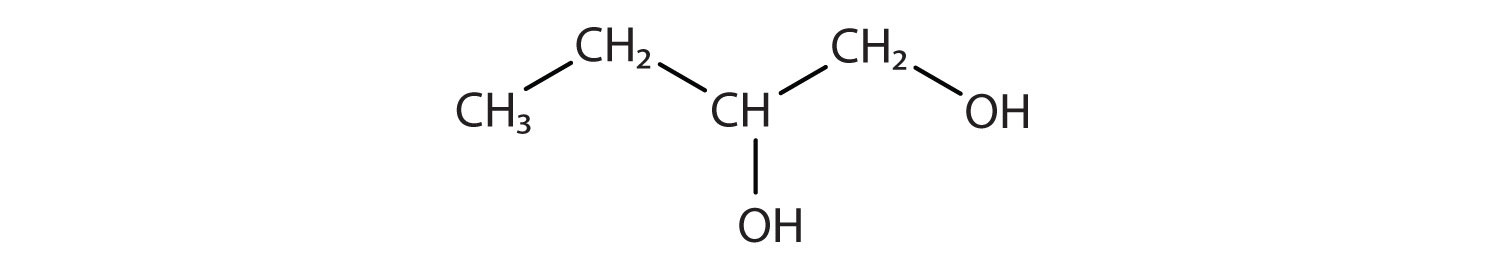
Identify the type of hydrocarbon in each structure.
-
-
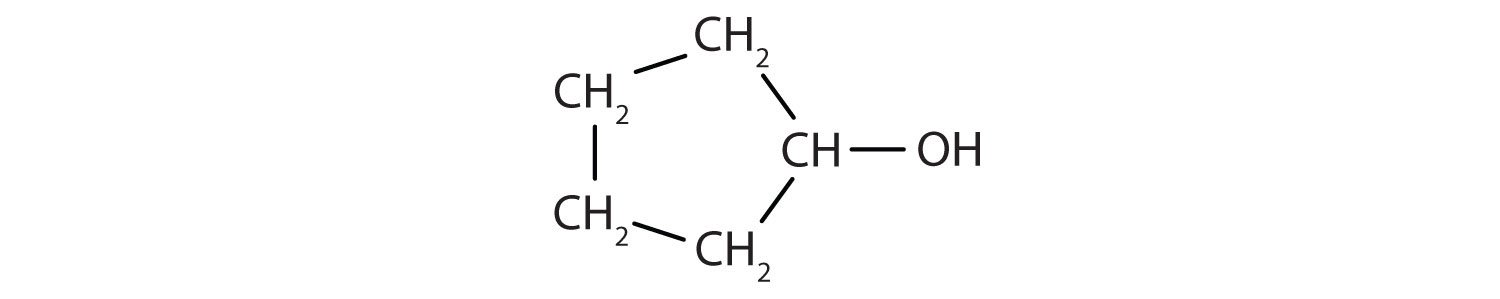
Identify the functional group(s) in each molecule.
-
-
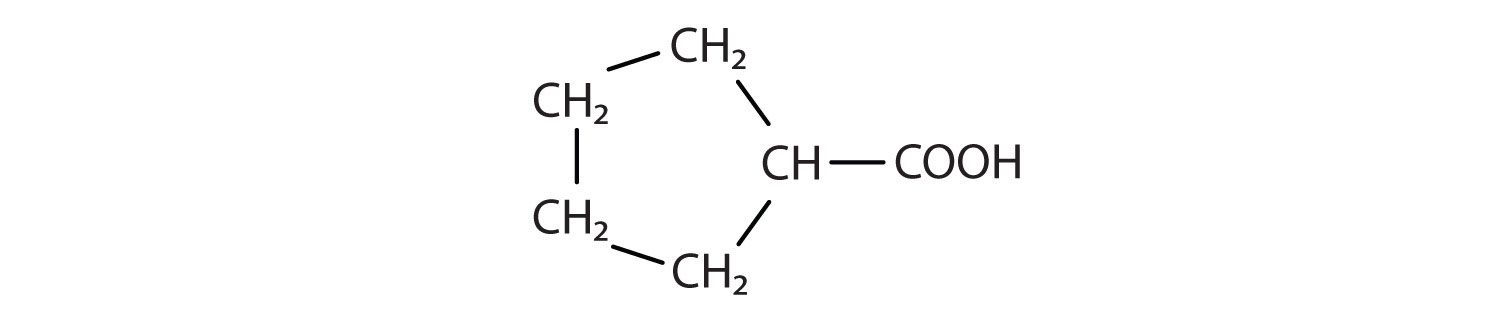
Identify the functional group(s) in each molecule.
-
-
How many functional groups described in this section contain carbon and hydrogen atoms only? Name them.
-
What is the difference in the ways the two oxygen atoms in the carboxyl group are bonded to the carbon atom?
Answers
-
Carbon atoms bond reasonably strongly with other carbon atoms. Carbon atoms bond reasonably strongly with atoms of other elements. Carbon atoms make a large number of covalent bonds (four).
-
- alkane
- alkene
- alkene
- alkyne
-
- alcohol
- carboxyl
- alcohol
- carbon-carbon double bond and carbon-carbon triple bond
-
two; carbon-carbon double bonds and carbon-carbon triple bonds